4.04 Membrane Lipids
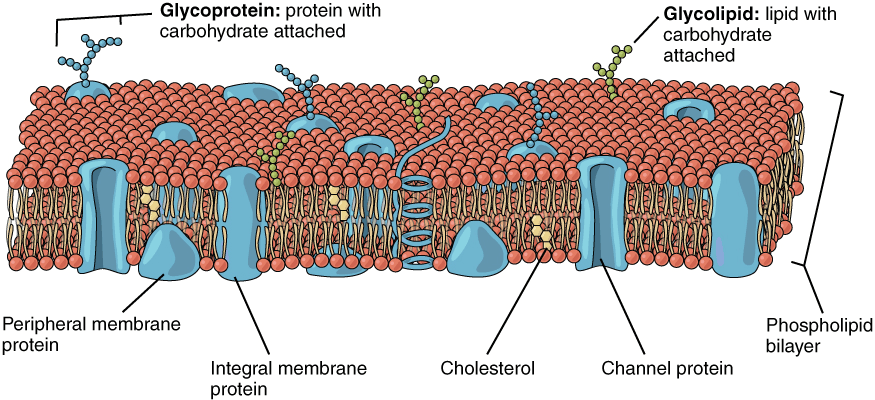
Overview of Membrane Lipids:
- Membrane lipids are a group of amphiphilic (having both hydrophilic and hydrophobic ends) compounds similar in structure to fats and oils.
- The three main classes of membrane lipids are:
- phospholipids,
- glycolipids,
- cholesterol.
- This amphiphilic nature allows lipids to form a bilayer, with hydrophilic (polar) ends facing outward towards the aqueous environments and hydrophobic (non-polar) ends facing inward, creating a separation between the cell’s internal and external environments.
Major Classes of Membrane Lipids:
- Phospholipids:
- Structure:
- Composed of two hydrophobic hydrocarbon (fatty acid) chains attached to a hydrophilic (polar) head group.
- Function:
- Forms the structural foundation of the lipid bilayer, providing a semi-permeable barrier that regulates molecule entry and exit.
- Structure:
- Glycolipids:
- Structure:
- Glycolipids consist of a hydrophobic tail of fatty acids with one or more sugar (glyco-) units attached to the head.
- Function:
- Key in cell recognition and signalling, contributing to cellular interactions.
- Structure:
- Cholesterol:
- Location:
- Found in animal cell membranes, interspersed among phospholipids.
- Function:
- Regulates Fluidity: Prevents close packing of phospholipids at moderate temperatures.
- Maintains Flexibility: Prevents membrane solidification at lower temperatures.
- Stabilizes Membrane Integrity: Reduces leakage of small molecules.
- Presence:
- Exclusive to eukaryotic cell membranes, absent in prokaryotic membranes.
- Location:
Fatty Acids in Membrane Lipids:
- Structure:
- Fatty acids in membrane lipids typically contain an even number of carbon atoms, ranging from 14 to 24, with 16- and 18-carbon fatty acids being the most common.
- Saturation:
- Fatty acids may be saturated (no double bonds) or unsaturated (with double bonds, usually in a cis configuration).
- Impact on Fluidity:
- The degree of unsaturation and chain length profoundly affect membrane fluidity.
- High levels of unsaturated fatty acids, such as linolenic acid (18-carbons with three double bonds), contribute to the fluidity of plant thylakoid membranes, maintaining flexibility even in cooler temperatures.
Functional and Structural Roles of Lipids:
Structural Matrix
- Role: Lipids provide a foundational structure (matrix) for membrane proteins and other molecules to embed, helping the cell maintain its shape and stability.
- Example: Phospholipids form a bilayer, creating a barrier that keeps cell contents in and external substances out, much like walls keep air inside a balloon.
Functional Roles of Lipids
1. Cell Growth and Adhesion
- Role: Lipids in the cell membrane help regulate how cells grow and attach to each other and surfaces.
- Example: Glycolipids, which are lipids with carbohydrate chains, assist cells in sticking to neighboring cells. This is crucial in tissues like skin, where cells need to stay attached tightly for protection.
2. Biosynthesis
- Role: Lipids are building blocks for synthesizing other biomolecules.
- Example: Cholesterol, a key membrane lipid, is used by cells to make steroid hormones like estrogen and testosterone, essential for various bodily functions.
3. Enzyme Activity
- Role: Lipids influence the function of enzymes that are attached to or embedded within the cell membrane.
- Example: Certain phospholipids can activate protein kinase C (an enzyme important for cell signaling), which regulates cell processes like growth and differentiation.
Membrane Dynamics:
- Lipid Mobility:
- Lipids are dynamic, allowing the membrane to remain fluid and adaptable to changes in temperature and environmental conditions.
- Non-Bilayer Forming Lipids:
- Some lipids, such as those found in plant thylakoids, typically form non-bilayer structures but can integrate into the bilayer with other lipids to create a stable membrane.
Cholesterol
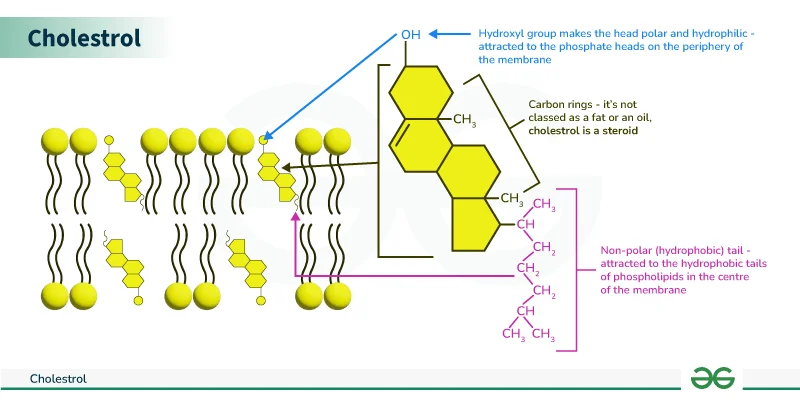
Cholesterol: Adds membrane stability and regulates fluidity, located within the bilayer among phospholipids.
Role in Fluidity:
- Regulates membrane fluidity by interacting with phospholipid tails:
- Prevents tight packing at low temperatures, avoiding membrane freezing and fracturing.
- Stabilizes the membrane at higher temperatures, preventing it from becoming overly fluid.
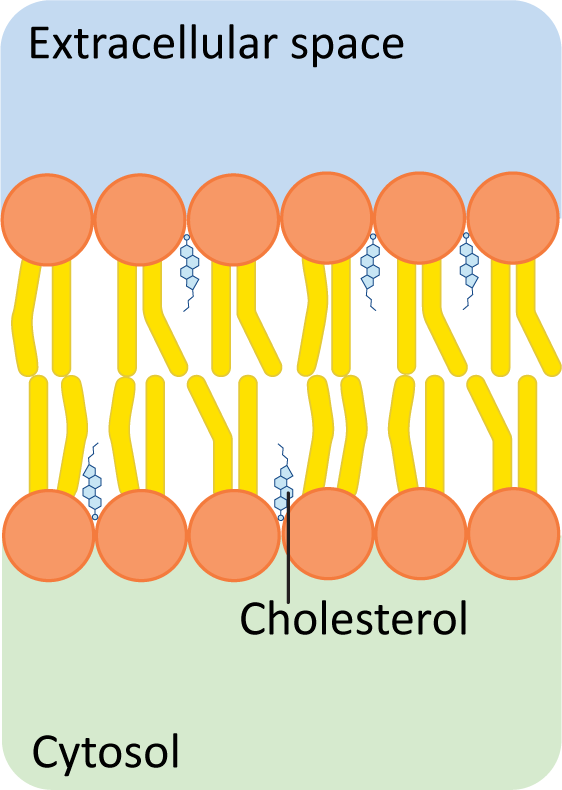
Structural Stability:
Binds to phospholipid tails, enhancing impermeability to ions and increasing the mechanical strength of the membrane.
Essential for membrane stability; without cholesterol, membranes could break down, leading to cell lysis.
Membrane Fluidity
Membrane Fluidity Factors
Adaptations to Temperature and Environment:
- Organisms’ Adaptations:
- Some organisms, like plants in cold climates or certain bacteria, adjust their membrane’s fatty acid balance to maintain functionality under varying temperatures.
- Temperature Influence:
- Lower temperatures reduce fluidity; membranes may solidify if too low.
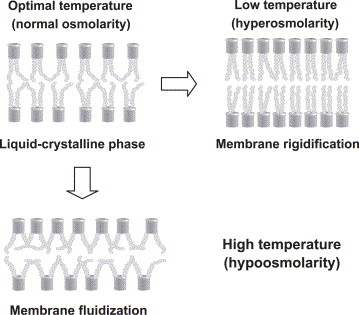
Membranes become less fluid when:
- High proportion of saturated fatty acids:
- Tight packing of straight chains leads to strong intermolecular forces.
- Low temperature:
- Molecules have reduced energy and pack closely together, increasing rigidity.
Membranes become more fluid when:
- Higher temperature:
- Increased molecular energy allows freer movement, enhancing fluidity.
- High proportion of unsaturated fatty acids:
- Bent chains reduce packing tightness and lower intermolecular forces.
Practise Questions
Question 1
Describe the amphiphilic nature of membrane lipids and explain its importance in forming the cell membrane. (5 marks)
Mark Scheme:
- Membrane lipids are amphiphilic, meaning they have both hydrophilic (water-attracting) and hydrophobic (water-repelling) regions. (1 mark)
- The hydrophilic head interacts with the aqueous environments inside and outside the cell. (1 mark)
- The hydrophobic tails avoid water and face inward, forming a nonpolar core. (1 mark)
- This arrangement forms a bilayer, providing a semi-permeable barrier that separates the cell’s internal and external environments. (1 mark)
- The bilayer allows for selective permeability, ensuring proper regulation of molecule entry and exit. (1 mark)
Question 2
Compare the structure and functions of phospholipids, glycolipids, and cholesterol in the cell membrane. (6 marks)
Mark Scheme:
- Phospholipids have two hydrophobic fatty acid tails and a hydrophilic phosphate head, forming the structural foundation of the bilayer. (1 mark)
- They regulate molecule entry and exit, maintaining selective permeability. (1 mark)
- Glycolipids have a hydrophobic tail and carbohydrate chains attached to their heads. (1 mark)
- They are involved in cell recognition and signalling, aiding cellular interactions. (1 mark)
- Cholesterol is embedded between phospholipids, stabilizing the membrane by regulating fluidity. (1 mark)
- Cholesterol prevents tight packing at low temperatures and excessive fluidity at high temperatures, ensuring membrane integrity. (1 mark)
Question 3
Explain the impact of saturated and unsaturated fatty acids on membrane fluidity. (5 marks)
Mark Scheme:
- Saturated fatty acids have straight chains that pack closely together, reducing membrane fluidity by increasing rigidity. (1 mark)
- Unsaturated fatty acids contain double bonds, creating kinks in their chains that prevent tight packing. (1 mark)
- These kinks increase the space between molecules, enhancing fluidity. (1 mark)
- At low temperatures, membranes with more unsaturated fatty acids remain fluid, preventing freezing. (1 mark)
- At high temperatures, saturated fatty acids stabilize the membrane, reducing excessive fluidity. (1 mark)
Question 4
What roles do cholesterol play in maintaining membrane stability and fluidity? (6 marks)
Mark Scheme:
- Cholesterol intersperses among phospholipids in the bilayer, interacting with their fatty acid tails. (1 mark)
- It prevents tight packing of phospholipids at low temperatures, maintaining fluidity and preventing freezing. (1 mark)
- At high temperatures, cholesterol reduces lipid movement, stabilizing the membrane. (1 mark)
- It enhances impermeability to small molecules and ions, strengthening the membrane. (1 mark)
- Cholesterol increases the mechanical strength of the membrane, preventing cell lysis. (1 mark)
- It is essential for maintaining membrane integrity, especially in animal cells. (1 mark)
Question 5
Explain how glycolipids contribute to cell recognition and adhesion. (5 marks)
Mark Scheme:
- Glycolipids consist of a hydrophobic fatty acid tail and a carbohydrate head group. (1 mark)
- The carbohydrate chains serve as recognition sites for other cells, aiding in cell-cell communication. (1 mark)
- Example: Glycolipids help the immune system differentiate between self and non-self cells. (1 mark)
- They assist in cell adhesion, allowing cells to bind together to form tissues. (1 mark)
- This is particularly important in maintaining the structural integrity of epithelial tissues. (1 mark)
Question 6
Describe how membrane lipids contribute to membrane dynamics and fluidity. (6 marks)
Mark Scheme:
- Membrane lipids, particularly phospholipids, are dynamic and can move laterally within the bilayer, ensuring fluidity. (1 mark)
- Unsaturated fatty acids in phospholipids increase fluidity by preventing tight packing. (1 mark)
- Cholesterol stabilizes the membrane, regulating fluidity by preventing excessive movement or solidification. (1 mark)
- Membrane fluidity allows the cell to adapt to changes in temperature and environment. (1 mark)
- It supports processes like endocytosis, exocytosis, and protein function within the membrane. (1 mark)
- The balance of saturated and unsaturated fatty acids ensures optimal membrane functionality. (1 mark)
Question 7
What are the functional roles of membrane lipids in enzyme activity and biosynthesis? (5 marks)
Mark Scheme:
- Membrane lipids influence enzymes embedded in or attached to the membrane, affecting their activity. (1 mark)
- Example: Certain phospholipids activate protein kinase C, regulating processes like cell growth and differentiation. (1 mark)
- Lipids are building blocks for synthesizing other biomolecules, such as steroid hormones. (1 mark)
- Example: Cholesterol is used to produce hormones like estrogen and testosterone. (1 mark)
- These roles make lipids crucial for cellular metabolism and signal transduction. (1 mark)
Question 8
How do fatty acid composition and membrane lipids help organisms adapt to varying temperatures? (6 marks)
Mark Scheme:
- Organisms adjust their membrane lipid composition to maintain fluidity under different temperatures. (1 mark)
- At low temperatures, a higher proportion of unsaturated fatty acids prevents membranes from solidifying. (1 mark)
- At high temperatures, saturated fatty acids stabilize the membrane, preventing excessive fluidity. (1 mark)
- Example: Plants in cold climates have membranes rich in unsaturated fatty acids, such as linolenic acid, to maintain flexibility. (1 mark)
- Certain bacteria adjust their fatty acid balance to survive extreme conditions. (1 mark)
- These adaptations ensure that cellular processes remain efficient regardless of environmental changes. (1 mark)
Question 9
Explain how the amphiphilic nature of lipids enables the formation of different structures in aqueous environments. (5 marks)
Mark Scheme:
- Amphiphilic lipids have hydrophilic heads that interact with water and hydrophobic tails that avoid water. (1 mark)
- In aqueous solutions, lipids self-assemble into structures like micelles, where tails face inward and heads face outward. (1 mark)
- Bilayers form when two layers of lipids arrange with tails facing inward and heads outward, creating a stable membrane. (1 mark)
- These structures allow cells to maintain distinct internal and external environments. (1 mark)
- The bilayer is the fundamental structure of the cell membrane, supporting its barrier and functional roles. (1 mark)
Question 10
Summarize the structural and functional roles of membrane lipids in maintaining cellular integrity. (6 marks)
Mark Scheme:
- Membrane lipids form a bilayer that provides structural stability to the cell. (1 mark)
- The hydrophobic core prevents leakage of essential molecules and blocks unwanted substances. (1 mark)
- Lipids contribute to fluidity, enabling membrane flexibility and adaptability. (1 mark)
- Cholesterol enhances stability, regulating fluidity and impermeability to ions. (1 mark)
- Functional lipids, such as glycolipids, aid in cell recognition, adhesion, and signalling. (1 mark)
- Lipids play a role in enzyme activity and biosynthesis, supporting cellular processes. (1 mark)