4.09 Osmosis
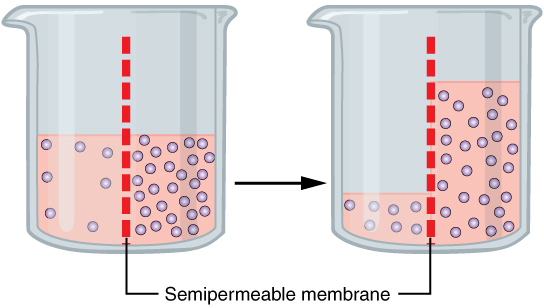
Definition:
- Osmosis is the net movement of water molecules across a partially permeable membrane from a region of higher water potential (dilute solution, high concentration of water) to a region of lower water potential (concentrated solution, low concentration of water).
- This movement aims to balance solute concentrations across the membrane.
Key Concepts in Osmosis
- Water Potential (ψ)
- Definition: Water potential is a measure of the tendency of water to move from one area to another. It helps to avoid confusion between water concentration and solute concentration.
- Pure Water: Has a water potential of 0 kPa, the highest possible water potential.
- Solutions with Solutes: Have negative water potentials. The more solute present, the more negative the water potential, indicating a lower tendency for water to leave the area.
- Movement in Osmosis: Water moves from an area of high water potential (less negative) to an area of low water potential (more negative).
- Partially Permeable Membrane: Allows small molecules, like water, to pass through but restricts larger solute molecules. This selective permeability enables osmosis to occur.
Mechanisms of Water Movement Across Membranes
- Lipid Bilayer: Although the lipid bilayer is generally impermeable to polar molecules, small water molecules can sometimes pass directly by squeezing between phospholipids.
- Aquaporins (Protein-lined Pores): Specialized channel proteins, or aquaporins, facilitate rapid and efficient water movement across the membrane, especially in cells requiring high water transport.
Factors Affecting Water Potential in Solutions
- Concentration of Solutes: Higher solute concentration lowers water potential, making it more negative.
- Applied Pressure: Adding mechanical pressure to a solution increases its water potential, encouraging water to move out.
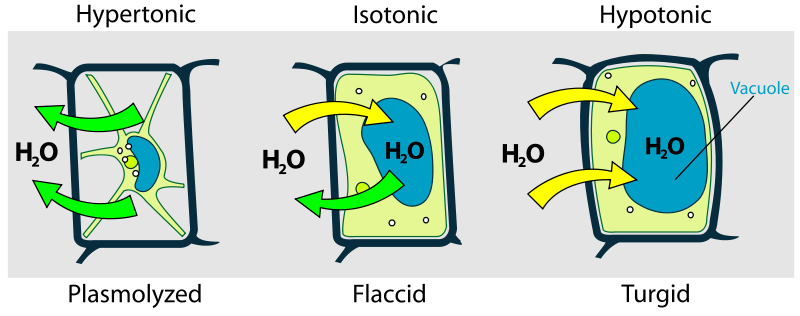

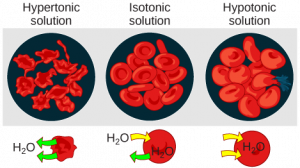
Osmosis in Cells
1. Osmosis in Animal Cells (e.g., red blood cells)
- Hypotonic Solution (higher water potential outside than inside the cell):
- Water enters the cell, causing it to swell and potentially burst, as animal cells lack a rigid cell wall.
- Isotonic Solution (equal water potential):
- No net movement of water; the cell maintains its shape, which is crucial for maintaining stable conditions in blood plasma and tissue fluid.
- Hypertonic Solution (lower water potential outside than inside the cell):
- Water exits the cell, causing it to shrink or become crenated (wrinkled).
2. Osmosis in Plant Cells
- Cell Wall Role: The rigid cell wall prevents plant cells from bursting in hypotonic environments.
- Key Terms:
- Turgid: A fully inflated plant cell, providing structural support when water has moved in by osmosis.
- Plasmolysis: In a hypertonic solution, water leaves the cell, causing the cytoplasm to shrink away from the cell wall.
- Incipient Plasmolysis: The point where the cell membrane begins to pull away from the wall, with no pressure on the cell wall.

Osmotic Pressure in Osmosis
Definition: Osmotic pressure is the pressure required to stop the movement of water across a semi-permeable membrane.
- Role in Osmosis: Osmotic pressure is a pulling force created by solute molecules in a solution. When two solutions of different solute concentrations are separated by a semi-permeable membrane, water moves towards the solution with a higher solute concentration (lower water potential).
- Mechanism: The greater the solute concentration, the higher the osmotic pressure, as more water molecules are “pulled” across the membrane toward the solution with higher osmotic pressure.
- Measurement: Osmotic pressure is proportional to the concentration of solutes in the solution. This pressure is measured in terms of how much force is needed to prevent water from moving across the membrane.
Example: In blood plasma, solutes like salts and proteins create osmotic pressure that draws water into capillaries from surrounding tissues.
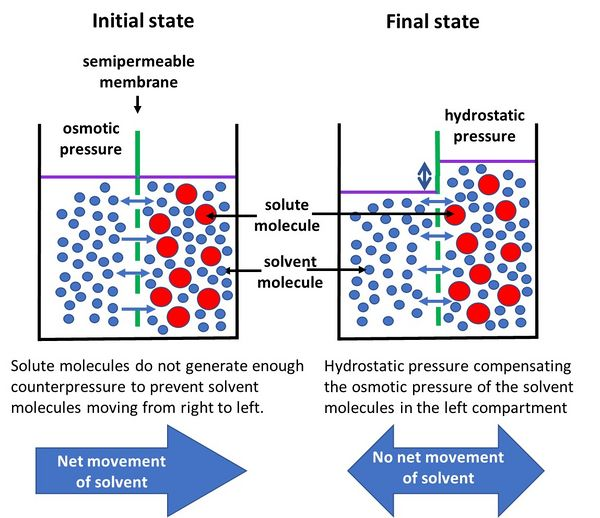
Hydrostatic Pressure in Osmosis
Definition: Hydrostatic pressure is the pressure exerted by a fluid in a confined space, such as water in a cell or a blood vessel.
- Role in Osmosis: Hydrostatic pressure is a pushing force exerted by the fluid itself. As water moves into an enclosed space (such as inside a cell or plant tissue), it increases the hydrostatic pressure within that space.
- Mechanism: When water enters a cell due to osmosis, it creates hydrostatic pressure within the cell. This pressure pushes outward against the cell membrane (and cell wall in plants), eventually counteracting the osmotic pressure and limiting further water influx.
- In Plants: In plant cells, the rigid cell wall resists excessive swelling by building up hydrostatic pressure, known as turgor pressure, which prevents additional water from entering and helps maintain cell structure.
Example: In blood vessels, hydrostatic pressure generated by the heart pushes water out of capillaries into surrounding tissues, opposing osmotic pressure.
Water Potential (Ψ) Formula
- Water Potential (Ψ) is the measure of the potential energy of water in a system, determining the direction of water movement. It combines two main components: solute potential (Ψs) and pressure potential (Ψp).
Water Potential Formula
Ψ = Ψs + Ψp
- Ψ (Water Potential): Represents the total potential energy of water, measured in kilopascals (kPa). Water moves from regions of higher (less negative) water potential to regions of lower (more negative) water potential.
- Ψs (Solute Potential): The component of water potential due to dissolved solutes. It is always negative in solutions because solutes lower the potential energy of water.
- Ψp (Pressure Potential): The component of water potential resulting from physical pressure on a solution. It can be positive (e.g., in plant cells due to turgor pressure) or zero in open systems like beakers.
Components Explained
- Solute Potential (Ψs):
- Effect: Solutes lower water potential by binding water molecules, reducing the free water available for movement.
- Calculation: The more solute present, the more negative the solute potential, as it decreases water potential.
- Example: In a sucrose solution, adding more sucrose makes the solution’s Ψs more negative, encouraging water to move into this solution from areas with higher water potential.
- Pressure Potential (Ψp):
- Effect: Physical pressure exerted on or by the water in a system.
- In Plant Cells: Turgor pressure (a type of pressure potential) builds up in plant cells as they fill with water, creating positive Ψp that counterbalances the inward pull of water from solute potential.
- In Open Systems: Pressure potential is zero (Ψp = 0), as there’s no confining boundary to exert pressure.
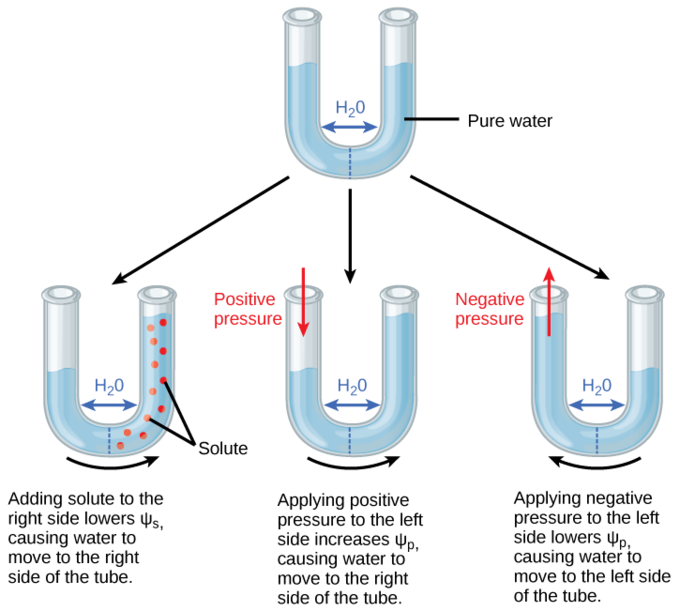
Examples of Calculating Water Potential
Example 1: Open Beaker of Sucrose Solution
- System: A beaker with a sucrose solution is open to the atmosphere, so Ψp = 0.
- Solute Potential (Ψs): Let’s say the solute potential of the sucrose solution is -200 kPa.
- Water Potential (Ψ):
Ψ = Ψs + Ψp = −200 kPa + 0 =−200 kPa - Interpretation: The water potential of the solution is -200 kPa. If placed next to pure water (Ψ = 0 kPa), water will move from the pure water (higher Ψ) into the sucrose solution (lower Ψ) due to osmosis.
Example 2: Plant Cell in a Hypotonic Solution
- System: A plant cell in a hypotonic (more dilute) solution, such as distilled water.
- Solute Potential (Ψs): Suppose the plant cell’s solute potential is -300 kPa.
- Pressure Potential (Ψp): As water enters the cell, turgor pressure builds up, so let’s say Ψp becomes +200 kPa.
- Water Potential (Ψ):
Ψ = Ψs + Ψp = −300 kPa + 200 kPa = −100 kPa - Interpretation: The overall water potential of the cell is -100 kPa. Since the water potential in distilled water (Ψ = 0 kPa) is higher, water will continue to enter the cell until Ψp increases enough to balance Ψs and reach equilibrium.
Example 3: Turgid Plant Cell
- System: A fully turgid plant cell, meaning it has reached equilibrium with the surrounding solution.
- Solute Potential (Ψs): Let’s assume the cell’s solute potential is -400 kPa.
- Pressure Potential (Ψp): The cell wall provides sufficient turgor pressure to counterbalance the solute potential, so Ψp = +400 kPa.
- Water Potential (Ψ):
Ψ = Ψs + Ψp = −400 kPa + 400 kPa = 0 kPa - Interpretation: The water potential of the cell is now 0 kPa, the same as the surrounding environment, so there is no net movement of water. The cell is in equilibrium and turgid, maintaining structural support.
Summary Table: Water Potential Components and Examples
| Example | Ψs (Solute Potential) | Ψp (Pressure Potential) | Ψ (Water Potential) | Water Movement |
|---|---|---|---|---|
| Open beaker with sucrose solution | -200 kPa | 0 kPa | -200 kPa | Water moves into the solution |
| Plant cell in hypotonic solution | -300 kPa | +200 kPa | -100 kPa | Water enters the cell |
| Fully turgid plant cell | -400 kPa | +400 kPa | 0 kPa | No net movement (equilibrium) |
Interaction Between Osmotic and Hydrostatic Pressure in Cells
- In cells, osmotic pressure and hydrostatic pressure interact to regulate water movement and maintain cellular balance:
- In Animal Cells:
- Hypotonic Solution: Water enters the cell due to higher osmotic pressure inside. As water accumulates, hydrostatic pressure builds up within the cell. Since animal cells lack a rigid cell wall, excessive hydrostatic pressure can cause the cell to burst (lysis).
- Isotonic Solution: The osmotic and hydrostatic pressures are balanced, so there is no net water movement, maintaining the cell’s shape and volume.
- Hypertonic Solution: Water leaves the cell, reducing internal hydrostatic pressure and causing the cell to shrink or crenate.
- In Plant Cells:
- Hypotonic Solution: Water enters the cell due to osmotic pressure, causing the cell to swell. However, the rigid cell wall resists excessive swelling by generating turgor pressure (a form of hydrostatic pressure), which eventually stops further water entry.
- Turgid Cell: When the hydrostatic (turgor) pressure inside a plant cell balances osmotic pressure, the cell becomes turgid, supporting the plant structure.
- Hypertonic Solution: Water leaves the plant cell, reducing hydrostatic pressure. The cell membrane pulls away from the cell wall in a process called plasmolysis.
Practical Application
- In capillaries, hydrostatic and osmotic pressures work together to regulate fluid exchange:
- Osmotic Pressure (from plasma proteins) draws water back into capillaries, maintaining fluid balance between blood and tissues.
- Hydrostatic Pressure (from heart pumping) pushes water and nutrients out of capillaries into tissues.
Summary Table: Osmotic vs. Hydrostatic Pressure
| Pressure Type | Role in Osmosis | Effect in Animal Cells | Effect in Plant Cells |
|---|---|---|---|
| Osmotic Pressure | Pulls water into solution with higher solute concentration | Drives water into the cell in hypotonic conditions | Draws water into the cell, but balanced by turgor pressure |
| Hydrostatic Pressure | Pushes water outward as fluid accumulates | Can cause cell lysis if too high | Builds up as turgor pressure, providing structural support |
Osmosis in Plants and Animals
ANIMALS

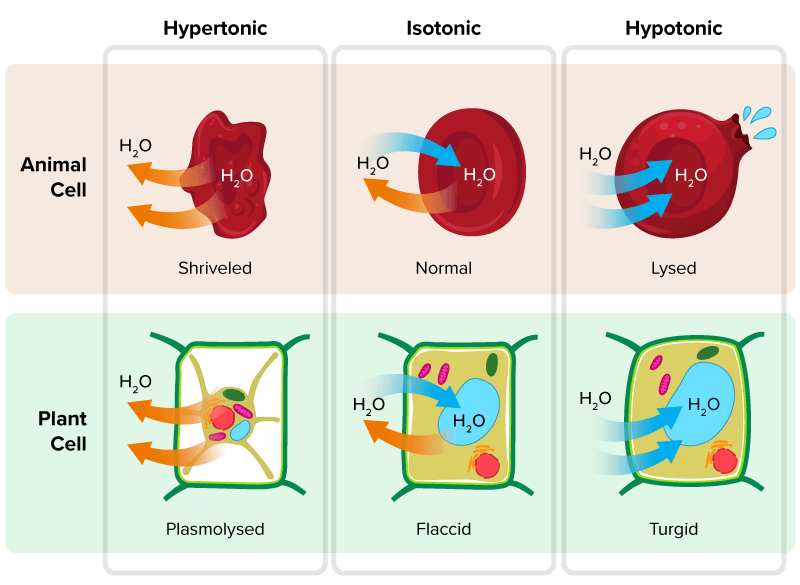
Hypertonic Solution (Higher Solute Concentration Outside the Cell)
- Water Movement: Water exits the red blood cells by osmosis because the solution outside the cell has a lower water potential (higher solute concentration) than the cell’s interior.
- Effect on Cells:
- The cells lose water, causing them to shrink and become shriveled.
- This shrinkage in red blood cells is known as crenation.
Isotonic Solution (Equal Solute Concentration Inside and Outside the Cell)
- Water Movement: There is no net movement of water because the water potential is balanced inside and outside the cell.
- Effect on Cells:
- Cells maintain their normal shape, as water movement in both directions is balanced.
- The cells retain a stable size and function effectively.
Hypotonic Solution (Lower Solute Concentration Outside the Cell)
- Water Movement: Water enters the red blood cells by osmosis because the external solution has a higher water potential (lower solute concentration).
- Effect on Cells:
- The cells swell as they absorb water.
- If the influx of water continues, the cells may burst (a process known as cytolysis).
Key Points
- No Cell Wall: Unlike plant cells, animal cells do not have a cell wall. This lack of structural support makes them more vulnerable to changes in water potential, leading to either bursting or shrinking in response to hypotonic or hypertonic solutions, respectively.
- Importance of Osmoregulation: Animal cells rely on maintaining an isotonic environment to prevent extreme water movement, which can damage cells and disrupt function.
- Exam Tip:
- Avoid using the term “plasmolysis” for animal cells, as this term applies only to plant cells with a cell wall.
- Use the term “crenation” for red blood cells or other animal cells that shrink due to water loss in a hypertonic solution.
PLANTS
Definition of Osmosis
- Osmosis is the net movement of water molecules from an area of higher water potential (dilute solution) to an area of lower water potential (concentrated solution) across a partially permeable membrane.
Osmosis in Hypotonic Solutions (Higher Water Potential Outside the Cell)
- Water Movement: When a plant cell is placed in pure water or a dilute solution, water enters the cell by osmosis, as the external solution has a higher water potential than the plant cell’s interior.
- Process:
- Water enters the vacuole, increasing the cell’s volume.
- The protoplast (the living part of the cell inside the cell wall) expands, pressing against the inelastic cell wall.
- This creates turgor pressure, an internal pressure that prevents further water from entering, stopping the cell from bursting.
- When fully expanded, the cell is turgid.
- Importance of Turgidity:
- Turgid cells keep the plant firm, providing structural support.
- Turgidity enables plants to stand upright, optimizing leaf position for photosynthesis.
- Without enough water, cells lose turgidity, causing the plant to wilt.
Osmosis in Hypertonic Solutions (Lower Water Potential Outside the Cell)
- Water Movement: When a plant cell is placed in a concentrated solution (with lower water potential than the cell), water leaves the cell by osmosis.
- Process:
- As water exits, the vacuole shrinks, causing the protoplast to retract from the cell wall.
- This process, called plasmolysis, results in a plasmolysed cell.
- Characteristics of Plasmolysis:
- The cell loses internal pressure, and the cell wall no longer experiences tension from the protoplast.
- The cell membrane pulls away from the cell wall, though the cell wall remains freely permeable, allowing external solutions to surround the protoplast.
Key Points
- Water Potential: Pure water has the highest water potential (0 kPa). All solutions have negative water potentials.
- Turgid Cells: Turgid cells have high internal pressure, providing support and rigidity to the plant.
- Plasmolysis: In plasmolysis, the protoplast shrinks and pulls away from the cell wall as the cell loses water.
Exam Advise:
- The cell wall is freely permeable and supports the cell’s shape, enabling external solutions to flow around the protoplast during plasmolysis.
- Cell Membrane vs. Cell Wall:
- The cell membrane is partially permeable, allowing selective movement of molecules based on size and polarity.
Osmosis in plants vs animals
Common Features:
- Cell Membrane: Both plant and animal cells have a cell membrane composed of a phospholipid bilayer, which is partially permeable, allowing osmosis to occur.
- Osmosis Occurs in Both: Water can move into and out of both plant and animal cells by osmosis.
Differences:
- Cell Wall:
- Plant Cells: Have a cell wall made of cellulose, which is fully permeable and provides structural support.
- Animal Cells: Lack a cell wall, making them more vulnerable to changes in osmotic pressure.
- Effect of Lower Water Potential Solution (Hypertonic Environment):
- Plant Cells: Water leaves the cell, causing the volume to decrease. The protoplast shrinks and pulls away from the cell wall, resulting in plasmolysis.
- Animal Cells: Water also leaves the cell, causing it to shrink and shrivel up. This is referred to as crenation in red blood cells.
- Effect of Higher Water Potential Solution (Hypotonic Environment):
- Plant Cells: Water enters, increasing the cell’s volume. The cell becomes turgid as the protoplast pushes against the cell wall. The cell wall withstands the pressure, preventing rupture.
- Animal Cells: Water enters, increasing the cell volume. Without a cell wall, the cell membrane stretches and may eventually burst (cytolysis) due to the lack of structural support.
Key Notes:
- Osmoregulation in Animals: Animal cells must maintain an isotonic environment to avoid cell bursting or shrinking due to osmotic pressure changes.
- Turgidity in Plants: Turgid cells provide structural support to plants, helping them maintain shape.
Practise Questions
Question 1
Define osmosis and explain the role of water potential in determining the direction of water movement. (4 marks)
Mark Scheme:
- Definition: Osmosis is the net movement of water molecules across a partially permeable membrane from a region of higher water potential (less negative) to a region of lower water potential (more negative). (1 mark)
- Water Potential: Water potential (Ψ) is the measure of the tendency of water to move from one area to another, with pure water having the highest Ψ (0 kPa). (1 mark)
- Direction of Movement: Water moves down its water potential gradient, from areas of higher Ψ to lower Ψ, until equilibrium is reached. (1 mark)
- Membrane Role: The partially permeable membrane allows water to pass but restricts solute movement. (1 mark)
Question 2
Describe how osmosis affects an animal cell placed in a hypotonic, isotonic, and hypertonic solution. (6 marks)
Mark Scheme:
- Hypotonic Solution:
- Water enters the cell due to higher Ψ outside. (1 mark)
- The cell swells and may burst (lysis) as it lacks a cell wall to resist hydrostatic pressure. (1 mark)
- Isotonic Solution:
- No net water movement as Ψ inside and outside the cell are equal. (1 mark)
- The cell retains its normal shape and volume. (1 mark)
- Hypertonic Solution:
- Water exits the cell due to lower Ψ outside. (1 mark)
- The cell shrinks and becomes crenated (wrinkled appearance). (1 mark)
Question 3
Explain the process and significance of plasmolysis in plant cells when placed in a hypertonic solution. (5 marks)
Mark Scheme:
- Water Movement: Water leaves the cell by osmosis, moving from higher Ψ inside to lower Ψ outside. (1 mark)
- Cell Shrinkage: The cytoplasm and vacuole shrink, and the protoplast pulls away from the cell wall. (1 mark)
- Definition of Plasmolysis: The process where the cell membrane detaches from the cell wall due to water loss. (1 mark)
- Effects on Cell: Loss of turgor pressure causes the cell to become flaccid, leading to wilting in plants. (1 mark)
- Reversibility: Plasmolysis can be reversed if the cell is placed in a hypotonic solution before significant damage occurs. (1 mark)
Question 4
Discuss the significance of turgor pressure in plant cells. (5 marks)
Mark Scheme:
- Definition of Turgor Pressure: The hydrostatic pressure exerted by water inside the vacuole against the cell wall. (1 mark)
- Structural Support: Provides rigidity to the plant cell, helping maintain cell shape. (1 mark)
- Upright Growth: Keeps non-woody plants upright and maintains leaf position for optimal photosynthesis. (1 mark)
- Prevention of Water Entry: High turgor pressure prevents further water influx, balancing osmotic and hydrostatic pressures. (1 mark)
- Role in Growth: Turgor pressure helps in cell elongation by stretching the cell wall during growth. (1 mark)
Question 5
Explain the role of water potential components (solute potential and pressure potential) in osmosis within plant cells. (6 marks)
Mark Scheme:
- Water Potential Formula: Ψ = Ψs + Ψp (1 mark)
- Solute Potential (Ψs):
- Always negative as solutes lower the potential energy of water. (1 mark)
- Determines the inward pull of water due to solute concentration. (1 mark)
- Pressure Potential (Ψp):
- Positive in plant cells due to turgor pressure exerted by the vacuole against the cell wall. (1 mark)
- Acts to resist further water entry, balancing the osmotic pull of solutes. (1 mark)
- Net Water Movement: Water moves into the cell until Ψs is balanced by Ψp, resulting in no net movement at equilibrium. (1 mark)
Question 6
A red blood cell is placed in pure water. Predict and explain the outcome. (4 marks)
Mark Scheme:
- Prediction: The red blood cell will swell and eventually burst (lysis). (1 mark)
- Reason: Pure water has a higher Ψ compared to the Ψ inside the cell, causing water to enter by osmosis. (1 mark)
- Lack of Cell Wall: Unlike plant cells, animal cells lack a rigid cell wall to counteract the osmotic pressure. (1 mark)
- Outcome: The increasing hydrostatic pressure within the cell causes the membrane to rupture. (1 mark)
Question 7
Explain how aquaporins enhance the efficiency of osmosis in cells. (4 marks)
Mark Scheme:
- Definition of Aquaporins: Specialized protein channels that facilitate water movement across the cell membrane. (1 mark)
- Increased Efficiency: Allow rapid and large-scale water transport compared to slow diffusion through the lipid bilayer. (1 mark)
- Cell Types: Found in cells requiring high water transport, such as kidney cells and root cells in plants. (1 mark)
- Selective Transport: Ensure selective water movement without allowing solutes or ions to pass. (1 mark)
Question 8
A plant cell is placed in a hypotonic solution. Explain the process and outcome. (5 marks)
Mark Scheme:
- Water Movement: Water enters the cell by osmosis, moving from higher Ψ outside to lower Ψ inside. (1 mark)
- Swelling of the Vacuole: The vacuole enlarges as it takes in water, increasing the cell’s internal volume. (1 mark)
- Turgor Pressure: The cell wall resists further expansion by exerting turgor pressure. (1 mark)
- Outcome: The cell becomes turgid, preventing further water entry and maintaining structural support. (1 mark)
- Importance: Turgidity provides mechanical support, helping the plant stand upright. (1 mark)