12.01 The Need for Energy in Living Organisms
Essential Cellular Processes Requiring Energy
- Active Transport
- Definition: The movement of substances across cell membranes against their concentration gradients.
- Importance: Enables cells to maintain concentration differences essential for functions like nutrient uptake and ion balance.
- Intracellular Movement
- Examples:
- Transporting proteins from ribosomes to the Golgi apparatus.
- Vesicle trafficking within the cell.
- Mechanism: Utilizes motor proteins (e.g., kinesin, dynein) that consume ATP to move along cytoskeletal tracks.
- Examples:
- Whole-Cell Movement
- Example: Muscle contraction.
- Mechanism: Actin and myosin filaments slide past each other, a process driven by ATP hydrolysis.
- Anabolic Reactions
- Definition: Building large molecules from smaller ones.
- Examples:
- DNA replication.
- Protein synthesis.
- Significance: Essential for growth, repair, and replication of cells.
ATP: The Universal Energy Currency
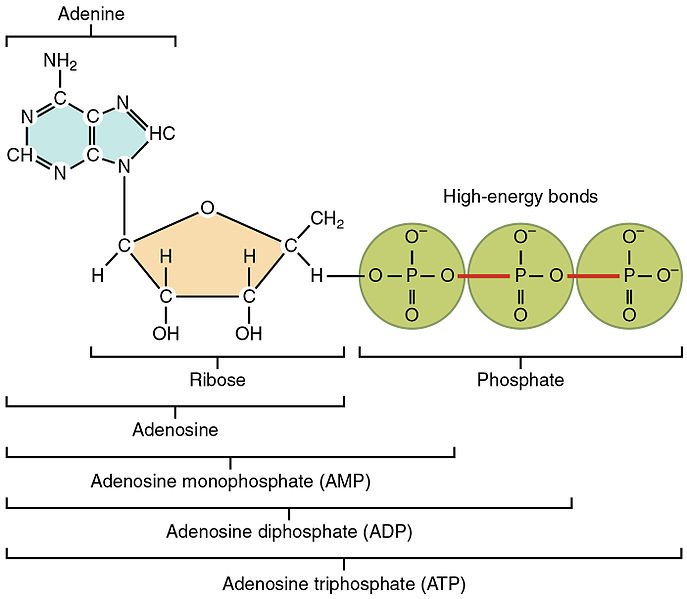
- Definition: ATP (adenosine triphosphate) is a phosphorylated nucleotide composed of:
- Adenine Base
- Ribose Sugar
- Three Phosphate Groups
Structure Overview:
- Figure A: Illustrates the structure of ATP, highlighting the adenine, ribose, and three phosphate groups.
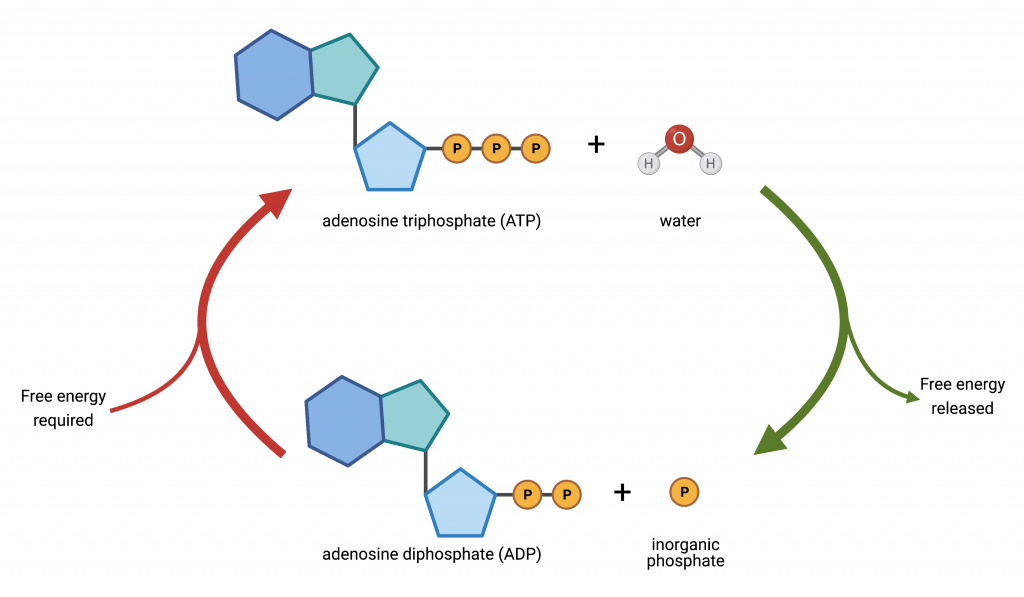
Energy Release through ATP Hydrolysis
- ATP releases energy by removing phosphate groups in a stepwise manner:
- ATP → ADP + Pi
- Energy Released: +30.5 kJ/mol
- ADP → AMP + Pi
- Energy Released: +30.5 kJ/mol
- AMP → Adenosine + Pi
- Energy Released: +14.2 kJ/mol
Figure B: Depicts the ATP hydrolysis reactions, showing the energy released at each step.
Benefits of ATP as an Energy Currency
- Immediate Energy Availability
- Mechanism: ATP hydrolysis occurs rapidly where and when energy is needed within the cell.
- Efficient Energy Usage
- Advantage: Provides just the right amount of energy to fuel cellular processes without excess waste.
- Stability
- Property: ATP is stable at the cell’s pH and requires specific enzymes (ATPases) for hydrolysis, preventing unintended energy release.
ATP Production
Continuous Synthesis:
- Necessity: Cells cannot store ATP in large quantities; hence, ATP must be synthesized continuously.
- Daily Usage: Approximately 50 kg of ATP is used daily by human cells.
Formation Methods:
- Substrate-Level Phosphorylation
- Process: A phosphate group is directly added to ADP using energy derived from another chemical reaction.
- Chemiosmosis
- Process: Hydrogen ions (protons) move down their concentration gradient across mitochondrial membranes, driving ATP synthesis via ATP synthase.
Respiration and ATP Synthesis
- In Humans:
- Pathways: ATP is synthesized through both substrate-level phosphorylation and chemiosmosis during cellular respiration.
- Stages of Respiration:
- Glycolysis.
- Krebs Cycle.
- Electron Transport Chain.
- In Plants:
- Additional Pathway: ATP is also produced during photosynthesis, supplementing respiration to meet energy demands.
- Refer to Chapter 13 for detailed mechanisms of photosynthetic ATP production.
Necessity of Respiration
- Breathing: Supplies oxygen required for cellular respiration.
- Role of Oxygen: Acts as the final electron acceptor in the electron transport chain, enabling the oxidation of glucose and efficient ATP synthesis.
Key Terms
- Anabolic: Processes that build larger molecules from smaller ones.
- Respiration: The enzymatic release of energy from organic compounds within cells.
- ATPase: Enzymes that catalyze the hydrolysis of ATP.
Discussion Questions
- Equation for ATP Formation:
- Reaction: ADP + Pi → ATP + H₂O
- Reference: Consult Figure 12.3 for a detailed depiction of this reaction.
Energy Currency:
- Discussion Point: Explain why ATP is referred to as the “energy currency” of the cell.
- Analogy: Just as money is exchanged to perform transactions, ATP is exchanged to transfer energy for various cellular processes.
Practice Questions
Question 1: Define Active Transport and explain its importance in cellular functions.
Marking Scheme:
Definition of Active Transport (2 marks):
- 1 mark: Correctly identifies active transport as the movement of substances across cell membranes against their concentration gradients.
- 1 mark: Mentions that it requires energy (implicitly understanding it uses ATP).
Importance in Cellular Functions (3 marks):
- 1 mark: States that active transport maintains concentration differences essential for cell function.
- 1 mark: Provides an example, such as nutrient uptake.
- 1 mark: Provides another example, such as maintaining ion balance.
Total: 5 marks
Sample Answer: Active transport is the movement of substances across cell membranes from areas of lower concentration to higher concentration, against their concentration gradients. This process is important because it allows cells to maintain concentration differences that are essential for functions like nutrient uptake and ion balance.
Question 2: Describe the mechanism of intracellular movement within a cell. Include the role of motor proteins and cytoskeletal tracks in your answer.
Marking Scheme:
Mechanism Description (4 marks):
- 1 mark: Mentions intracellular movement involves transporting substances like proteins and vesicles.
- 1 mark: Identifies motor proteins such as kinesin and dynein.
- 1 mark: Explains that motor proteins consume ATP to function.
- 1 mark: Describes movement along cytoskeletal tracks (e.g., microtubules).
Additional Details for Partial Credit:
- Correct identification of examples (transporting proteins, vesicle trafficking).
- Linking ATP consumption to the movement process.
Total: 4 marks
Sample Answer: Intracellular movement involves transporting proteins from ribosomes to the Golgi apparatus and vesicle trafficking within the cell. This process utilizes motor proteins like kinesin and dynein, which consume ATP to move along cytoskeletal tracks such as microtubules, enabling the directed movement of cellular components.
Question 3: Explain how ATP is utilized during muscle contraction.
Marking Scheme:
Process Explanation (4 marks):
- 1 mark: Identifies that muscle contraction involves actin and myosin filaments.
- 1 mark: States that these filaments slide past each other.
- 1 mark: Mentions that ATP hydrolysis provides the energy for this process.
- 1 mark: Connects ATP hydrolysis to the sliding mechanism driven by actin and myosin.
Additional Details for Partial Credit:
- Correctly linking ATP to the movement of actin and myosin.
Total: 4 marks
Sample Answer: During muscle contraction, actin and myosin filaments slide past each other. This sliding mechanism is driven by the hydrolysis of ATP, which provides the necessary energy for the myosin heads to pull the actin filaments, resulting in muscle shortening and contraction.
Question 4: What are anabolic reactions? Provide two examples and explain their significance in cells.
Marking Scheme:
Definition of Anabolic Reactions (1 mark):
- Correctly states that anabolic reactions build large molecules from smaller ones.
Examples (2 marks):
- 1 mark: DNA replication.
- 1 mark: Protein synthesis.
Significance Explanation (2 marks):
- 1 mark: Essential for growth.
- 1 mark: Necessary for repair and replication of cells.
Total: 5 marks
Sample Answer: Anabolic reactions are processes that build large molecules from smaller ones. Examples include DNA replication and protein synthesis. These reactions are significant because they are essential for the growth, repair, and replication of cells, allowing organisms to develop and maintain their structures.
Question 5: Describe the structure of ATP, including its main components.
Marking Scheme:
Structure Description (4 marks):
- 1 mark: Adenine base.
- 1 mark: Ribose sugar.
- 1 mark: Three phosphate groups.
- 1 mark: Overall mention of ATP being a phosphorylated nucleotide.
Total: 4 marks
Sample Answer: ATP, or adenosine triphosphate, is a phosphorylated nucleotide composed of an adenine base, a ribose sugar, and three phosphate groups. This structure allows ATP to store and transfer energy within cells.
Question 6: Explain the process of ATP hydrolysis and the energy released at each step.
Marking Scheme:
Process Explanation (4 marks):
- 1 mark: ATP hydrolysis involves the removal of phosphate groups.
- 1 mark: ATP → ADP + Pi releases +30.5 kJ/mol.
- 1 mark: ADP → AMP + Pi releases +30.5 kJ/mol.
- 1 mark: AMP → Adenosine + Pi releases +14.2 kJ/mol.
Energy Details (2 marks):
- Correct values for energy released at each step.
Total: 6 marks
Sample Answer: ATP hydrolysis involves the stepwise removal of phosphate groups, releasing energy at each stage. First, ATP is converted to ADP and inorganic phosphate (Pi), releasing +30.5 kJ/mol. Next, ADP is converted to AMP and Pi, also releasing +30.5 kJ/mol. Finally, AMP is converted to adenosine and Pi, releasing +14.2 kJ/mol. This stepwise release of energy powers various cellular processes.
Question 7: Why is ATP referred to as the “universal energy currency” of the cell?
Marking Scheme:
Immediate Energy Availability (1 mark):
- ATP hydrolysis occurs rapidly where and when energy is needed.
Efficient Energy Usage (1 mark):
- Provides the right amount of energy without excess waste.
Stability (1 mark):
- ATP is stable at the cell’s pH and requires specific enzymes for hydrolysis.
Summary (1 mark):
- ATP effectively transfers energy to fuel cellular processes.
Total: 4 marks
Sample Answer: ATP is called the “universal energy currency” of the cell because it provides immediate energy availability by hydrolyzing rapidly where and when needed. It ensures efficient energy usage by supplying just the right amount of energy for cellular processes without excess waste. Additionally, ATP is stable under cellular conditions and requires specific enzymes for hydrolysis, preventing unintended energy release. This makes ATP an effective medium for energy transfer within the cell.
Question 8: Compare substrate-level phosphorylation and chemiosmosis in ATP production.
Marking Scheme:
Substrate-Level Phosphorylation (2 marks):
- Direct addition of a phosphate group to ADP.
- Uses energy from a chemical reaction.
Chemiosmosis (2 marks):
- Involves hydrogen ions moving down their concentration gradient.
- Drives ATP synthesis via ATP synthase.
Comparison (2 marks):
- Both produce ATP.
- Different mechanisms: direct transfer vs. proton gradient-driven.
Total: 6 marks
Sample Answer: Substrate-level phosphorylation involves the direct addition of a phosphate group to ADP, using energy derived from another chemical reaction. In contrast, chemiosmosis involves hydrogen ions moving down their concentration gradient across mitochondrial membranes, which drives ATP synthesis through the enzyme ATP synthase. While both processes result in the production of ATP, they utilize different mechanisms: substrate-level phosphorylation transfers a phosphate group directly, whereas chemiosmosis relies on a proton gradient to generate ATP.
Question 9: Outline the stages of cellular respiration in humans and indicate where ATP is synthesized.
Marking Scheme:
Stages Identification (3 marks):
- Glycolysis.
- Krebs Cycle.
- Electron Transport Chain.
ATP Synthesis Locations (3 marks):
- Glycolysis: Substrate-level phosphorylation.
- Krebs Cycle: Substrate-level phosphorylation.
- Electron Transport Chain: Chemiosmosis.
Total: 6 marks
Sample Answer: The stages of cellular respiration in humans include glycolysis, the Krebs cycle, and the electron transport chain. ATP is synthesized during glycolysis and the Krebs cycle through substrate-level phosphorylation, where phosphate groups are directly transferred to ADP. In the electron transport chain, ATP is produced via chemiosmosis, where hydrogen ions move down their concentration gradient through ATP synthase, driving the synthesis of ATP.
Question 10: Explain the role of oxygen in cellular respiration.
Marking Scheme:
Oxygen’s Role in Respiration (3 marks):
- Acts as the final electron acceptor in the electron transport chain.
- Enables the oxidation of glucose.
- Facilitates efficient ATP synthesis by allowing the electron transport chain to continue.
Connection to ATP Production (2 marks):
- Without oxygen, the electron transport chain cannot function, stopping ATP production via chemiosmosis.
Total: 5 marks
Sample Answer: Oxygen plays a crucial role in cellular respiration by acting as the final electron acceptor in the electron transport chain. This allows electrons to flow through the chain, enabling the oxidation of glucose and the continuation of ATP synthesis. Without oxygen, the electron transport chain would halt, preventing the production of ATP via chemiosmosis and ultimately disrupting the cell’s energy supply.