10.07 Practicals
Testing for Antibiotic Sensitivity
Understanding how bacteria respond to different antibiotics is crucial for effective treatment and managing antibiotic resistance. Antibiotic Sensitivity Testing determines the susceptibility of bacteria to various antibiotics, guiding healthcare professionals in selecting the most appropriate treatment.
Purpose of Antibiotic Sensitivity Testing
- Determine Effective Antibiotics: Identify which antibiotics can effectively inhibit or kill a specific bacterial strain.
- Guide Treatment Decisions: Assist clinicians in choosing the most suitable antibiotic therapy for infections.
- Monitor Resistance Patterns: Track the prevalence and spread of antibiotic-resistant bacteria in different environments.
Antibiotic Sensitivity Test Overview
The Antibiotic Sensitivity Test assesses how bacteria respond to various antibiotics. The most common method used is the Disk Diffusion Method, also known as the Kirby-Bauer Test.
Disk Diffusion Method (Kirby-Bauer Test)
Materials and Equipment Required
- Bacterial Culture: Pure strain of the bacteria to be tested.
- Agar Plates: Typically Mueller-Hinton agar is used for standardized results.
- Antibiotic-Impregnated Disks: Small, circular paper disks saturated with specific antibiotics.
- Sterile Inoculating Loop: For spreading bacteria evenly on the agar plate.
- Incubator: Maintains optimal temperature (usually 37°C) for bacterial growth.
- Ruler or Caliper: For measuring inhibition zones.
- Gloves and Lab Coat: For personal protection and maintaining sterility.
- Forceps or Disk Dispenser: For placing antibiotic disks on the agar.
Procedure
- Preparation of Bacterial Culture:
- Growth Phase: Use bacteria in the mid-logarithmic (log) phase to ensure active growth.
- Standardization: Adjust the bacterial suspension to match the 0.5 McFarland standard (~1×1081 \times 10^81×108 CFU/mL) for consistent inoculum density.
- Inoculation of Agar Plate:
- Spreading Bacteria: Using a sterile inoculating loop, dip into the standardized bacterial suspension.
- Creating a Lawn: Streak the entire surface of a Mueller-Hinton agar plate evenly to form a uniform bacterial lawn.
- Application of Antibiotic Disks:
- Placement: Using sterile forceps or a disk dispenser, place antibiotic-impregnated disks onto the surface of the inoculated agar.
- Spacing: Ensure disks are evenly spaced (typically 24 mm center-to-center) to prevent overlapping inhibition zones.
- Incubation:
- Conditions: Invert the agar plates to prevent condensation and incubate at 37°C for 18-24 hours.
- Environment: Maintain optimal humidity and temperature to support bacterial growth.
- Observation and Measurement:
- Inhibition Zones: After incubation, observe clear zones around antibiotic disks where bacterial growth has been inhibited.
- Measurement: Measure the diameter of each inhibition zone in millimeters using a ruler or caliper.
- Interpretation of Results:
- Comparison to Standards: Compare inhibition zone diameters to standardized charts (e.g., CLSI or EUCAST guidelines) to classify bacteria as:
- Sensitive (S): Inhibition zone diameter ≥ set threshold.
- Intermediate (I): Inhibition zone diameter between set thresholds.
- Resistant (R): Inhibition zone diameter ≤ set threshold.
- Comparison to Standards: Compare inhibition zone diameters to standardized charts (e.g., CLSI or EUCAST guidelines) to classify bacteria as:
Interpreting Inhibition Zones
- Clear Zones (Zones of Inhibition):
- Large Clear Zone: Indicates sensitivity; the antibiotic effectively inhibits bacterial growth.
- Small or No Clear Zone: Suggests resistance; the antibiotic is ineffective against the bacteria.
- Example Interpretation:
- Penicillin:
- Sensitive: Zone diameter ≥ 20 mm.
- Intermediate: Zone diameter between 15-19 mm.
- Resistant: Zone diameter ≤ 14 mm.
- Penicillin:
Example Practical Observation
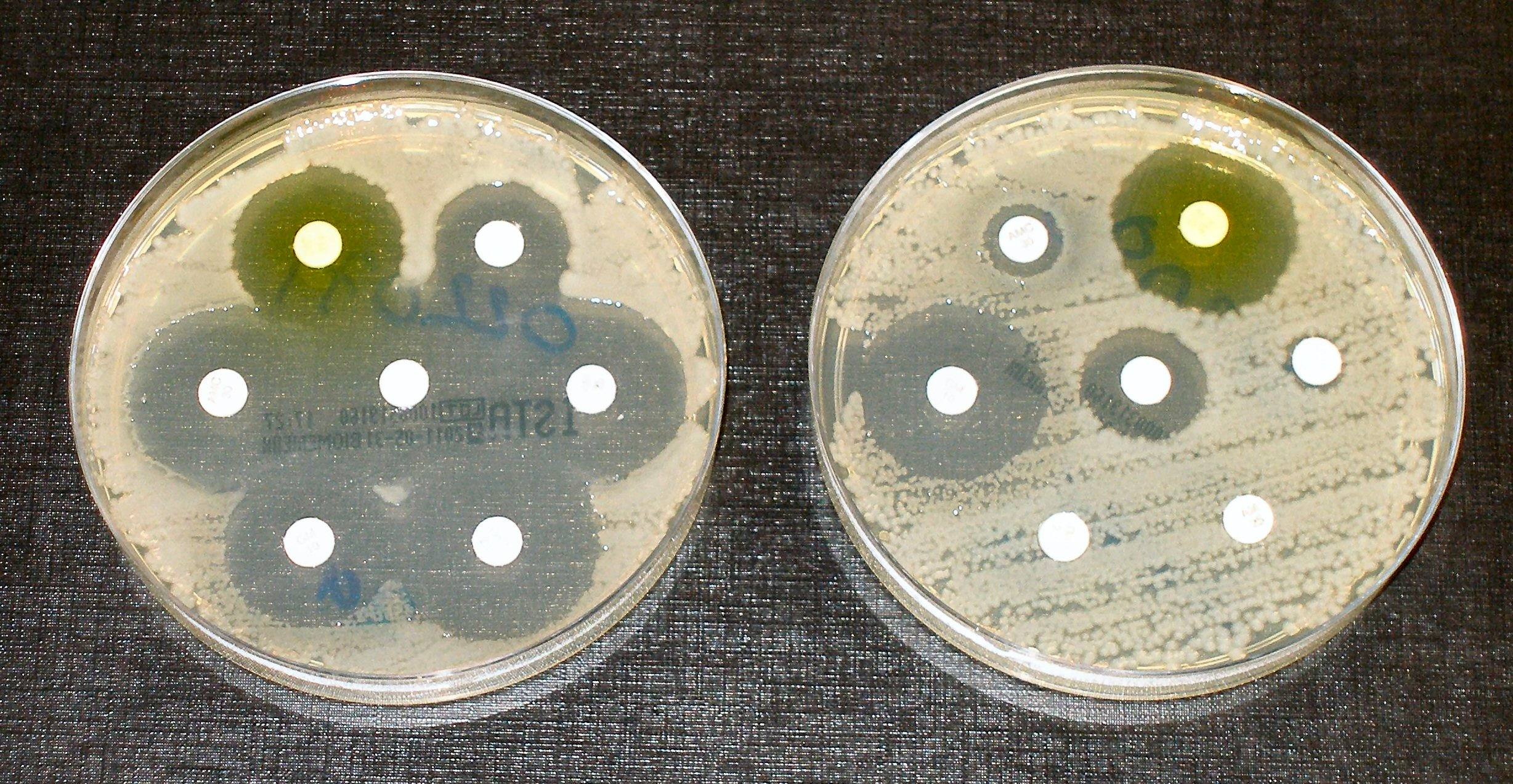
- Scenario:
- Left Plate: Clear rings around all antibiotic disks indicate that bacteria are not resistant to any of the antibiotics tested.
- Right Plate: Bacteria show full resistance to three antibiotics (no clear zones) and partial resistance to two antibiotics (smaller clear zones).
- Conclusion:
- Effective Antibiotics: Those with clear zones are suitable for treating the infection.
- Ineffective Antibiotics: Those with no or small clear zones should be avoided due to bacterial resistance.
Advantages of the Disk Diffusion Method
- Simplicity and Cost-Effectiveness: Requires minimal equipment and is easy to perform.
- Standardization: Widely accepted protocols ensure consistency across different laboratories.
- Visual Results: Clear zones provide straightforward interpretation of bacterial sensitivity.
Limitations of the Disk Diffusion Method
- Qualitative Results: Provides categories (S, I, R) but not exact bacterial counts.
- Time-Consuming: Requires overnight incubation, delaying results.
- Influence of Variables: Agar thickness, incubation temperature, and time can affect zone sizes.
- Limited Scope: Not suitable for all bacterial species or antibiotics.
Applications of Antibiotic Sensitivity Testing
- Clinical Diagnostics: Guides the selection of appropriate antibiotic therapy for patients.
- Epidemiological Surveillance: Monitors the emergence and spread of antibiotic-resistant bacteria in communities and healthcare settings.
- Pharmaceutical Research: Assesses the efficacy of new antibiotics or modifications to existing ones.
- Public Health Policy: Informs strategies to combat antibiotic resistance through appropriate antibiotic use.
Safety Considerations
- Personal Protective Equipment (PPE): Always wear gloves, a lab coat, and eye protection to prevent exposure to pathogens and chemicals.
- Aseptic Technique: Maintain sterility to avoid contamination of samples and ensure accurate results.
- Proper Disposal: Dispose of used agar plates and antibiotic disks following biohazard waste protocols to prevent environmental contamination and the spread of resistance genes.