03.07 Enzyme Action: Substrate Concentration
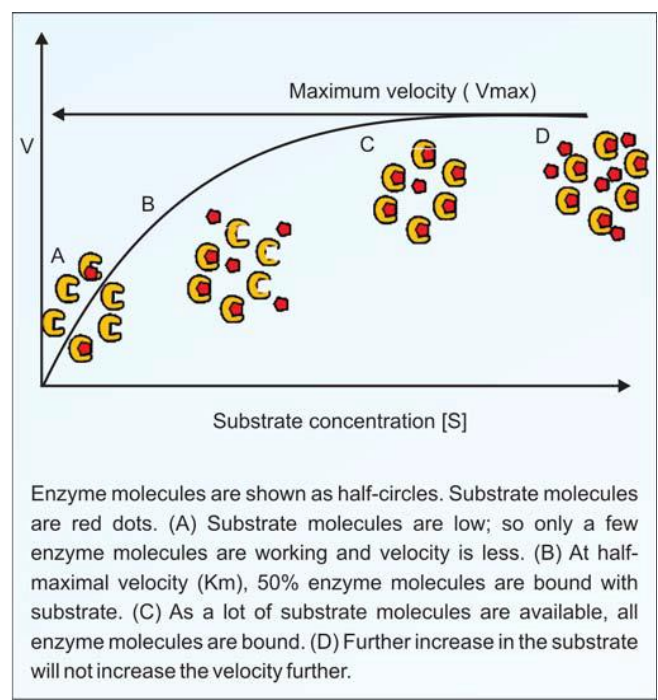
Overview of Substrate Concentration on Reaction Rate
- Experiment Setup:
- Enzyme: Catalase (constant concentration).
- Substrate: Hydrogen peroxide (H₂O₂).
- Procedure:
- Varied amounts of hydrogen peroxide were used while keeping catalase concentration constant.
- Measured oxygen production (product) and plotted oxygen volume over time.
- Initial rate of reaction calculated for the first 30 seconds for each concentration.
Observations and Graph Interpretation
- Relationship between Substrate Concentration and Initial Rate:
- At Low Substrate Concentrations:
- Initial rate of reaction increases as substrate concentration increases.
- Explanation: More substrate molecules mean more frequent collisions with enzyme active sites, leading to more reactions.
- At High Substrate Concentrations:
- Reaction rate reaches a maximum point (Vmax), where increasing substrate concentration no longer increases reaction rate.
- Reason: All enzyme active sites are occupied, meaning the enzyme is working at full capacity.
- Any additional substrate molecules must wait for active sites to become available.
- At Low Substrate Concentrations:
Key Concept: Vmax (Maximum Velocity)
- Definition: Vmax represents the maximum rate of an enzyme-catalyzed reaction when all active sites are occupied.
- At Vmax, the enzyme is saturated, meaning the rate is only limited by enzyme concentration or speed of enzyme turnover.
Graph Characteristics

Initial Linear Increase:
- Direct proportionality between substrate concentration and reaction rate at low substrate levels.
Plateau at Vmax:
- Rate levels off as substrate concentration continues to increase.
- Explanation: All enzyme molecules are saturated with substrate; additional substrate does not increase rate.

Practical Implications
Enzyme Efficiency:
- To achieve maximum reaction rates, it is essential to have sufficient substrate concentration but not so much that resources are wasted beyond Vmax.
Practise Questions
Question 1
Explain how substrate concentration affects the rate of an enzyme-catalyzed reaction at low and high substrate levels. (5 marks)
Mark Scheme:
- At low substrate concentrations, the reaction rate increases as substrate concentration increases. (1 mark)
- Explanation: More substrate molecules mean more frequent collisions with enzyme active sites. (1 mark)
- At high substrate concentrations, the rate levels off and reaches a maximum (Vmax). (1 mark)
- Explanation: All enzyme active sites are occupied, and the enzyme is working at full capacity. (1 mark)
- Further increases in substrate concentration do not affect the rate because additional substrate must wait for active sites to become available. (1 mark)
Question 2
Define Vmax and explain its significance in enzyme-catalyzed reactions. (4 marks)
Mark Scheme:
- Vmax is the maximum rate of an enzyme-catalyzed reaction when all active sites are saturated with substrate. (1 mark)
- At Vmax, the enzyme is working at its full capacity. (1 mark)
- It indicates the maximum efficiency of the enzyme under the given conditions. (1 mark)
- Beyond Vmax, reaction rate is limited by enzyme concentration or the speed of enzyme turnover. (1 mark)
Question 3
Describe the shape of a graph showing reaction rate vs. substrate concentration and explain its features. (6 marks)
Mark Scheme:
- The graph initially shows a linear increase in reaction rate with increasing substrate concentration. (1 mark)
- This is because more substrate molecules increase the likelihood of collisions with active sites. (1 mark)
- The graph then levels off, forming a plateau at Vmax. (1 mark)
- At Vmax, all enzyme active sites are occupied, and the enzyme is saturated with substrate. (1 mark)
- Increasing substrate concentration beyond this point does not increase the reaction rate. (1 mark)
- The plateau illustrates that the rate is now limited by the enzyme’s capacity to process substrate. (1 mark)
Question 4
Outline an experiment to investigate the effect of substrate concentration on the rate of catalase activity. (6 marks)
Mark Scheme:
- Prepare solutions with varying concentrations of hydrogen peroxide (substrate). (1 mark)
- Use a constant concentration of catalase enzyme in each reaction mixture. (1 mark)
- Add the enzyme to the substrate solution and measure the volume of oxygen gas produced over a fixed time (e.g., 30 seconds) using a gas syringe. (1 mark)
- Record the initial rate of reaction for each substrate concentration. (1 mark)
- Plot a graph of reaction rate (Y-axis) against substrate concentration (X-axis). (1 mark)
- Control variables: Temperature, pH, and reaction volume to ensure accurate results. (1 mark)
Question 5
Why does the reaction rate plateau at high substrate concentrations in an enzyme-catalyzed reaction? (4 marks)
Mark Scheme:
- At high substrate concentrations, all enzyme active sites are occupied. (1 mark)
- The enzyme is saturated and working at its maximum capacity (Vmax). (1 mark)
- Additional substrate molecules must wait for active sites to become available, so they do not increase the reaction rate. (1 mark)
- The reaction rate is now limited by the enzyme concentration or turnover rate. (1 mark)
Question 6
Explain the importance of calculating the initial rate of reaction when investigating substrate concentration effects. (5 marks)
Mark Scheme:
- The initial rate reflects enzyme activity before substrate depletion begins. (1 mark)
- At the start, substrate concentration is consistent across all samples. (1 mark)
- Differences in reaction rate are solely due to substrate concentration, avoiding interference from limiting factors. (1 mark)
- The initial rate ensures a reliable comparison of enzyme efficiency under different conditions. (1 mark)
- Measuring the rate later in the reaction would lead to variability due to changes in substrate concentration. (1 mark)
Question 7
How does substrate concentration influence enzyme efficiency in practical applications? (5 marks)
Mark Scheme:
- At low substrate concentrations, reaction rates are low, leading to inefficient use of enzymes. (1 mark)
- Increasing substrate concentration improves efficiency by ensuring active sites are fully utilized. (1 mark)
- Maximum efficiency is achieved at Vmax, where all active sites are occupied. (1 mark)
- Adding more substrate beyond Vmax is wasteful, as it does not increase the reaction rate. (1 mark)
- In industry, ensuring the substrate concentration is sufficient but not excessive optimizes cost-effectiveness. (1 mark)
Question 8
Describe how the rate of an enzyme-catalyzed reaction changes with substrate concentration and explain why. (6 marks)
Mark Scheme:
- At low substrate concentrations, the rate increases proportionally with substrate concentration. (1 mark)
- Explanation: More substrate molecules increase the frequency of enzyme-substrate collisions. (1 mark)
- As substrate concentration rises further, the increase in rate begins to slow. (1 mark)
- At high substrate concentrations, the rate plateaus at Vmax. (1 mark)
- Explanation: Enzyme active sites are fully occupied, and the enzyme is saturated. (1 mark)
- Additional substrate does not affect the rate because enzymes cannot process more than their turnover capacity. (1 mark)
Question 9
What happens to the reaction rate if substrate concentration increases when enzymes are already saturated? (3 marks)
Mark Scheme:
- When enzymes are saturated, the reaction rate has reached Vmax and cannot increase further. (1 mark)
- Additional substrate molecules are unable to bind until active sites become available. (1 mark)
- The reaction rate remains constant regardless of further increases in substrate concentration. (1 mark)
Question 10
What conclusions can be drawn from a graph of reaction rate vs. substrate concentration? (5 marks)
Mark Scheme:
- At low substrate concentrations, reaction rate is proportional to substrate concentration, indicating enzyme activity depends on substrate availability. (1 mark)
- As substrate concentration increases, the reaction rate begins to slow, indicating active sites are becoming occupied. (1 mark)
- The rate plateaus at Vmax, showing the enzyme is saturated and working at maximum capacity. (1 mark)
- Beyond Vmax, the rate does not increase with additional substrate, as it is limited by enzyme concentration. (1 mark)
- The graph illustrates the relationship between enzyme efficiency, substrate availability, and saturation point. (1 mark)