03.06 Summary Chapter
1. Chemical Formulae
a. Molecular Formulae
- Definition: Represents the actual number of atoms of each element in a molecule.
- Representation: Uses chemical symbols from the Periodic Table with subscripts indicating the number of atoms.
- Diatomic Elements: Seven elements exist as diatomic molecules (X₂): Hydrogen (H₂), Nitrogen (N₂), Oxygen (O₂), Fluorine (F₂), Chlorine (Cl₂), Bromine (Br₂), and Iodine (I₂).
b. Empirical Formulae
- Definition: Simplest whole-number ratio of atoms in a compound.
- Difference from Molecular Formula: Often simpler; for ionic compounds, empirical and molecular formulae are identical.
- Example:
- Ethanoic Acid:
- Molecular Formula: C₂H₄O₂
- Empirical Formula: CH₂O
- Ethanoic Acid:
c. Ionic Compounds
- Formation: Metals lose electrons to form cations; non-metals gain electrons to form anions.
- Formula Determination:
- Direct Comparison: Balances charges directly.
- Swap-and-Drop Method: Swap the charge numbers of ions and drop the signs.
- Example:
- Iron(II) Sulfate: FeSO₄
- Copper(II) Chloride: CuCl₂
d. Hydrated Salts
- Definition: Ionic salts containing water molecules of crystallization.
- Formula Representation: CuSO₄·5H₂O (5 water molecules)
- Determination: By heating to remove water and calculating the ratio of water to salt.
2. Relative Masses
a. Relative Atomic Mass (Ar)
- Definition: Average mass of atoms of an element relative to carbon-12.
- Usage: Found on the Periodic Table.
- Example:
- Carbon (C): Ar = 12
- Oxygen (O): Ar = 16
b. Relative Molecular Mass (Mr)
- Definition: Sum of relative atomic masses of all atoms in a molecule.
- Calculation: Add the Ar of each element multiplied by the number of atoms in the molecule.
- Example:
- Water (H₂O): Mr = (2×1) + 16 = 18
3. The Mole Concept
a. The Mole & Avogadro’s Constant
- Mole (mol): Unit measuring the amount of substance.
- Avogadro’s Constant: 1 mole = 6.02 × 10²³ particles (atoms, molecules, ions).
- Molar Mass: Mass of 1 mole of a substance (g/mol).
b. Molar Volume of Gas
- Avogadro’s Law: Equal moles of gases occupy equal volumes at the same temperature and pressure.
- Molar Volume at RTP (20°C, 1 atm): 24 dm³/mol.
- Conversions:
- Volume (dm³) = Moles × 24
- Volume (cm³) = Moles × 24,000
c. Linking Moles, Mass & Mr
- Formula:
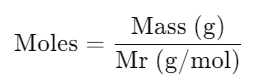
- Usage: Calculate mass from moles or vice versa using the molar mass.
d. Limiting Reactants
- Definition: Reactant that is completely consumed first, limiting the amount of product formed.
- Determination Steps:
- Convert masses to moles.
- Use the balanced equation to find mole ratios.
- Identify the reactant that produces the least product.
4. Concentration of Solutions
a. Units of Concentration
- Mass-Based: g/dm³
- Mole-Based: mol/dm³
b. Calculating Concentration
- Mass-Based Formula:
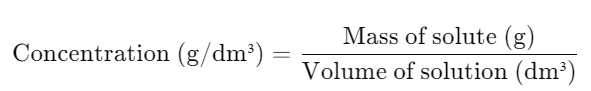
- Mole-Based Formula:
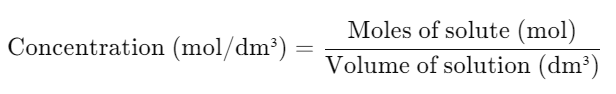
- Conversions:
- 1 dm³ = 1000 cm³
- Convert units as necessary.
c. Titration Calculations
- Purpose: Determine the concentration of an unknown solution by reacting it with a solution of known concentration.
- Steps:
- Write and balance the reaction equation.
- Calculate moles of known solution.
- Use mole ratio to find moles of unknown.
- Calculate concentration of unknown.
5. Reacting Masses
a. Law of Conservation of Mass
- Statement: Mass of reactants = Mass of products.
- Application: Ensures balanced chemical equations.
b. Calculations Involving Masses
- Steps:
- Balance the chemical equation.
- Convert masses to moles.
- Use mole ratios to find moles of desired substance.
- Convert moles back to mass using Mr.
c. Example Calculation
- Reaction: 2Ca + O2 → 2CaO
- Given: 40 g Ca + 32 g O₂ → 80 g CaO
- Verification: 80 g Ca + 32 g O₂ = 112 g CaO (Note: Ensure correct mole ratios in real calculations)
6. Percentage Calculations
a. Percentage Yield
- Definition:

- Importance: Measures efficiency of a reaction.
- Example:
- Actual Yield = 1.6 g
- Theoretical Yield = 2.0 g
- Percentage Yield:
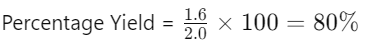
b. Percentage Composition
- Definition: Mass of each element as a percentage of the total mass of the compound.
- Formula:
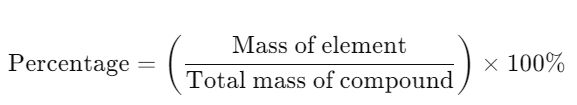
- Example:
- Water (H₂O):
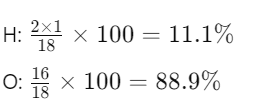
c. Percentage Purity
- Definition:
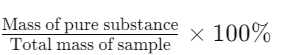
- Example:
- Pure Substance = 13.5 g
- Total Sample = 15.0 g
- Percentage Purity:
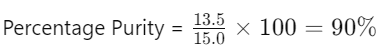
7. Empirical & Molecular Formulae
a. Calculating Empirical Formula
- Steps:
- Convert mass of each element to moles.
- Divide all mole values by the smallest number of moles.
- If necessary, multiply to obtain whole numbers.
- Example:
- Given: 10 g H and 80 g O
- Moles: H = 10/1 = 10; O = 80/16 = 5
- Ratio: H₂O
b. Calculating Molecular Formula
- Steps:
- Find the empirical formula.
- Calculate Mr of empirical formula.
- Divide Mr of molecular formula by Mr of empirical formula to find multiplier.
- Multiply subscripts in empirical formula by multiplier.
- Example:
- Empirical Formula: C₄H₁₀S
- Mr Empirical: 90
- Mr Molecular: 180
- Multiplier: 180/90 = 2
- Molecular Formula: C₈H₂₀S₂
8. Balancing Chemical Equations
a. Importance
- Ensures the Law of Conservation of Mass is followed.
- Equal number of each type of atom on both sides.
b. Methods
- Trial and Error: Adjust coefficients to balance atoms.
- Mole Ratio: Use the coefficients from the balanced equation for calculations.
c. Example
- Unbalanced: Al + CuO → Al₂O₃ + Cu
- Balanced: 2Al + 3CuO → Al₂O₃ + 3Cu
Exam Tips & Tricks
- Always Balance Equations: Double-check atom counts on both sides.
- Show All Workings: Even if the final answer is incorrect, partial credit may be awarded.
- Use Formula Triangles: Helpful for memorizing relationships between concentration, moles, and volume.
- Check Units: Ensure consistency, especially when converting between cm³ and dm³.
- Understand Concepts: Memorization helps, but understanding application is crucial for problem-solving.
Worked Examples
1. Determining the Empirical Formula
- Problem: 10 g H and 80 g O in a compound.
- Solution:
- Moles H = 10/1 = 10
- Moles O = 80/16 = 5
- Ratio H:O = 10:5 = 2:1
- Empirical Formula: H₂O
2. Calculating Percentage Composition
- Problem: Fe₂O₃ composition.
- Solution:
- Mass Fe = 2 × 56 = 112 g
- Mass Fe₂O₃ = 2×56 + 3×16 = 160 g
- % Fe = (112/160) × 100 = 70%
3. Balancing a Reaction
- Problem: Al burns in Cl₂ to form AlCl₃.
- Balanced Equation: 2Al + 3Cl₂ → 2AlCl₃
4. Titration Calculation
- Problem: 25.0 cm³ HCl titrated with 12.1 cm³ 0.100 mol/dm³ NaOH.
- Solution:
- Moles NaOH = 0.0121 dm³ × 0.100 mol/dm³ = 0.00121 mol
- Moles HCl = 0.00121 mol (1:1 ratio)
- Concentration HCl = 0.00121 mol / 0.025 dm³ = 0.0484 mol/dm³