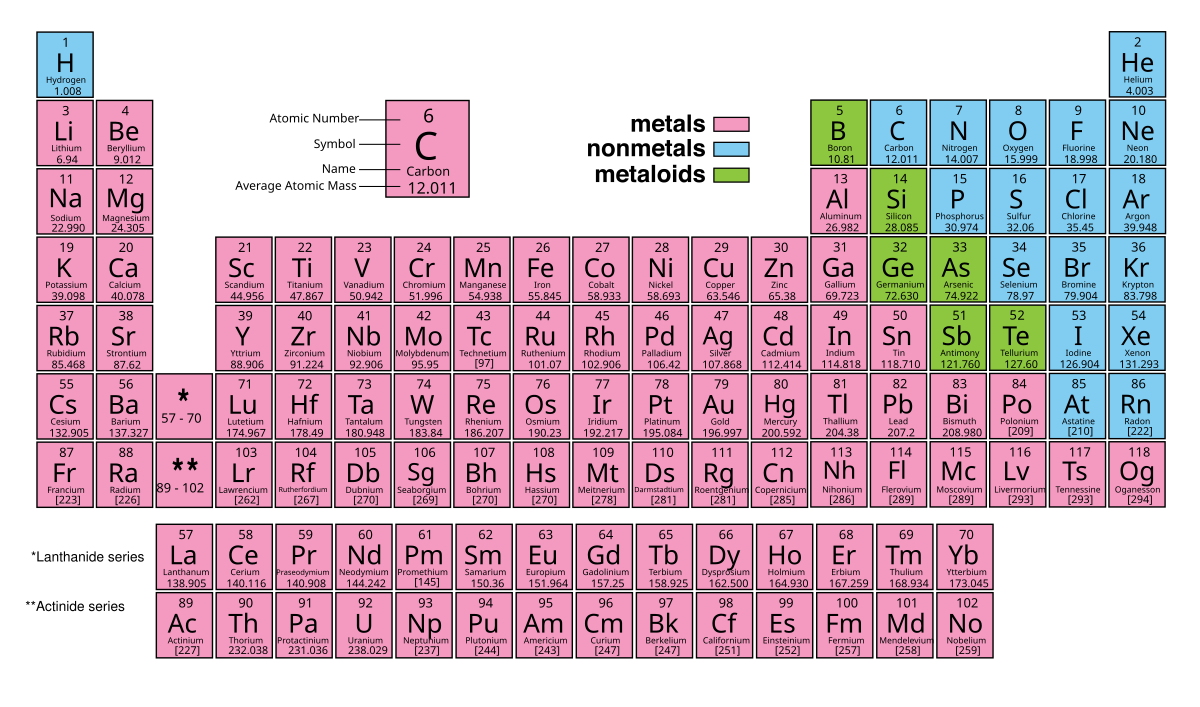
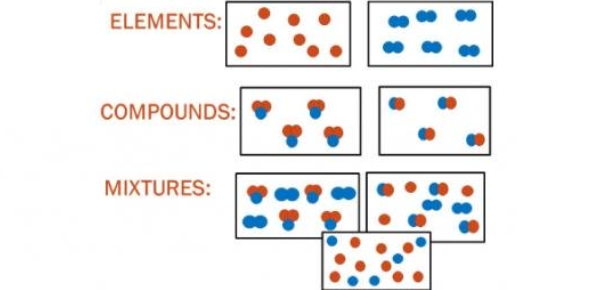
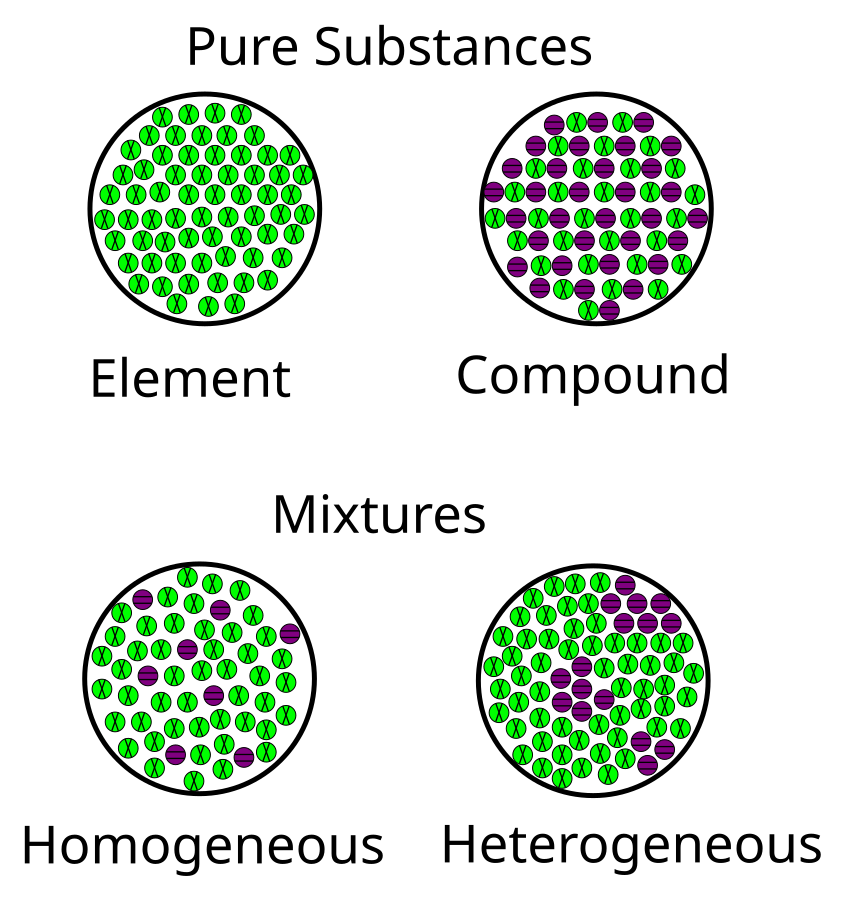
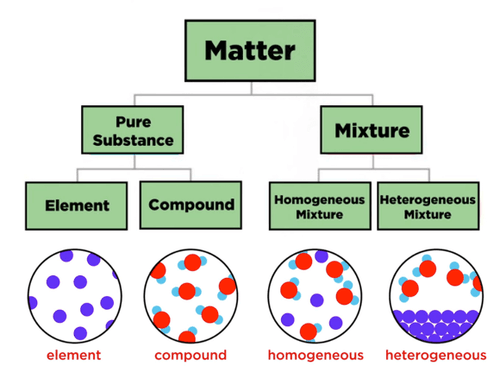
03.01 Elements, Compounds, and Mixtures
a. Elements
Definition:
- A pure substance that cannot be broken down into simpler substances by chemical methods.
Characteristics:
- Unique Properties: Each element has its own distinct properties (e.g., gold is shiny and malleable, while sulfur is yellow and brittle).
- Number of Elements:
- Over 100 known elements
- 94 occur naturally (e.g., Hydrogen, Oxygen, Carbon)
- Classification:
- Metals: Conduct electricity, are shiny, malleable (e.g., Iron, Copper)
- Non-Metals: Poor conductors, diverse properties (e.g., Oxygen, Nitrogen)
- Atomic Number: All atoms of an element have the same number of protons.
- Example: All carbon atoms have 6 protons.
Examples of Elements:
- Hydrogen (H)
- Carbon (C)
- Gold (Au)
- Oxygen (O₂)
b. Compounds
Definition:
- A substance formed when two or more elements chemically combine in fixed proportions.
Characteristics:
- Unique Properties: Compounds have properties different from their constituent elements.
- Example: Sodium (a reactive metal) + Chlorine (a poisonous gas) → Sodium Chloride (table salt, edible)
- Chemical Formula: Indicates the elements in a compound and their ratios.
- Examples:
- Water: H₂O (2 Hydrogen atoms + 1 Oxygen atom)
- Carbon Dioxide: CO₂ (1 Carbon atom + 2 Oxygen atoms)
- Examples:
Types of Compounds:
- Molecular (Covalent) Compounds:
- Formation: Atoms share electrons.
- Examples:
- Water (H₂O)
- Ammonia (NH₃)
- Methane (CH₄)
- Ionic Compounds:
- Formation: Transfer of electrons creates ions, which attract each other.
- Examples:
- Sodium Chloride (NaCl)
- Calcium Carbonate (CaCO₃)
c. Mixtures
Definition:
- A combination of two or more substances (elements or compounds) that are not chemically bonded.
Characteristics:
- Variable Composition: The ratio of components can change.
- Example: Air can have varying amounts of nitrogen, oxygen, and other gases.
- Properties: Each component retains its individual properties.
- Example: In a salad, lettuce remains crunchy, and tomatoes remain juicy.
Types of Mixtures:
- Solutions (Homogeneous Mixtures):
- Definition: Uniform composition throughout.
- Examples:
- Saltwater (Salt dissolved in water)
- Vinegar
- Heterogeneous Mixtures:
- Definition: Non-uniform composition; components can be seen separately.
- Examples:
- Sand and Water
- Air (mixture of gases)
- Alloys like Brass (Copper + Zinc)
Separation Methods:
- Physical Techniques:
- Filtration: Separates solids from liquids (e.g., tea leaves from tea).
- Magnetism: Uses magnetic properties to separate materials (e.g., iron filings from sand).
- Distillation: Separates based on boiling points (e.g., separating alcohol from water).
d. Comparison: Compounds vs. Mixtures
| Property | Compounds | Mixtures |
|---|---|---|
| Nature | Single substance | Two or more substances |
| Composition | Fixed proportions | Variable proportions |
| Formation | Chemical reactions | Physical combination |
| Properties | Different from constituent elements | Retain individual properties |
| Separation | Requires chemical reactions to separate | Can be separated by physical methods |
Additional Examples for Better Understanding
- Elements:
- Metals:
- Iron (Fe): Used in construction and manufacturing.
- Copper (Cu): Used in electrical wiring.
- Non-Metals:
- Nitrogen (N₂): Makes up about 78% of Earth’s atmosphere.
- Sulfur (S): Used in fertilizers and chemicals.
- Metals:
- Compounds:
- Water (H₂O): Essential for life, used in various chemical reactions.
- Carbon Dioxide (CO₂): Produced by respiration and combustion, used by plants in photosynthesis.
- Sodium Chloride (NaCl): Common table salt used in cooking and preserving food.
- Mixtures:
- Homogeneous:
- Air: A mixture of nitrogen, oxygen, argon, and other gases.
- Brass: An alloy of copper and zinc.
- Heterogeneous:
- Trail Mix: A mixture of nuts, dried fruits, and seeds.
- Oil and Water: Do not mix uniformly.
- Homogeneous:
5. Key Terminology
- Element: A substance made of only one type of atom (e.g., Oxygen).
- Compound: A substance formed from two or more elements in fixed ratios (e.g., Water – H₂O).
- Mixture: A combination of two or more substances not chemically bonded (e.g., Air).
- Covalent Bonding: Sharing of electron pairs between atoms (e.g., H₂O).
- Diatomic Molecule: A molecule composed of two atoms (e.g., O₂, N₂).
- Chemical Formula: A representation of a compound using symbols and numerical ratios (e.g., CO₂).
- Dot-and-Cross Diagram: A diagram showing electron sharing in covalent bonds.
- Intermolecular Forces: Forces between molecules, such as hydrogen bonds or dipole-dipole interactions.
- Intramolecular Forces: Forces within a molecule, primarily covalent bonds.
Examples
Question 1:
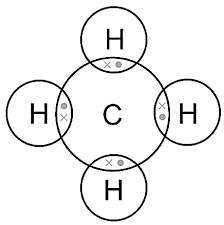
Covalent bonding involves electrons being shared between the atoms bonded together. Methane is made up of covalently bonded molecules. Which diagram represents the bonding in methane?
Options:
- Diagram A: Carbon atom at the center with four hydrogen atoms each sharing a pair of electrons.
A)
B)
- Diagram B: Sodium and chlorine ions arranged in a lattice structure.
Answer:
- Methane (CH₄): Carbon shares electrons with four hydrogen atoms through single covalent bonds.
Correct Selection: Diagram A
Explanation:
Methane has a central carbon atom bonded to four hydrogen atoms by sharing electrons, forming a tetrahedral shape. Diagram B represents an ionic compound (like NaCl), not a covalent molecule.