12.11 Past Paper Practise
Question 1
Explain why carbohydrates, lipids and proteins have different relative energy values as substrates in respiration in aerobic conditions. [6]
any six from
1 different substrates have different numbers of, hydrogens / C-H bonds ;
2 lipids have (relatively) more, hydrogens / C-H bonds (than carbohydrates or proteins) ;
3 hydrogens / C-H bonds, located in fatty acid (tails of lipids) ;
4 breakdown / oxidation, of substrate provides hydrogen (atoms) ;
5 for reduction of, NAD / FAD ;
6 (reduced, NAD / FAD) provides / releases, hydrogen to ETC ;
7 hydrogen (dissociates) into protons and electrons ;
8 ref. energy used to set up proton gradient ;
9 chemiosmosis / oxidative phosphorylation / AW ;
10 (so) more, ATP / energy, from lipids per unit mass (than, carbohydrates / proteins) or lipids, more energy dense / have
higher (relative) energy value ;
Define the term respiratory quotient (RQ) and describe how you would carry out an investigation to determine the RQ of germinating barley seeds. [9]
RQ
1 (ratio of) carbon dioxide given out divided by oxygen taken in ;
2 ref. volume / moles ;
R amount
3 per unit time ;
any eight from investigation
4 use respirometer ;
5 seeds placed on, mesh / gauze ;
6 KOH / NaOH / sodalime, to absorb carbon dioxide ;
7 manometer / capillary tube / syringe ;
8 movement of fluid (in manometer / capillary tube / syringe) = uptake of oxygen ;
9 keep, temperature / air pressure, constant ;
10 measure oxygen uptake after certain time ;
11 repeat without KOH / NaOH / sodalime ;
12 difference in manometer readings due to carbon dioxide given out ;
Question 2
Complete the following passage.
During strenuous exercise, muscles often do not receive sufficient oxygen to support aerobic respiration. As a result, muscles carry out ………………………………………..respiration and produce ……………………………………….. , which diffuses into the blood.
Most is then absorbed by the ……………………………………….. , which respires it to form carbon dioxide and water or uses it to form glucose. The volume of oxygen absorbed by the lungs does not return to normal immediately after strenuous exercise because the
body has to repay an oxygen ……………………………………….. . Exercise that uses the cardiovascular and gaseous exchange systems is termed ……………………………………….. exercise. Improvements in fitness of the cardiovascular system can be followed by measuring the decrease in the ……………………………………….. pulse rate. [6] [Total : 6]

Fig. 2.1 is an electron micrograph of a mitochondrion.

Two stages of respiration occur in mitochondria. These are the Krebs cycle and oxidative phosphorylation.
(a) Complete the table below by naming the structures labelled A and B and stating which of the stages of respiration occur in each.

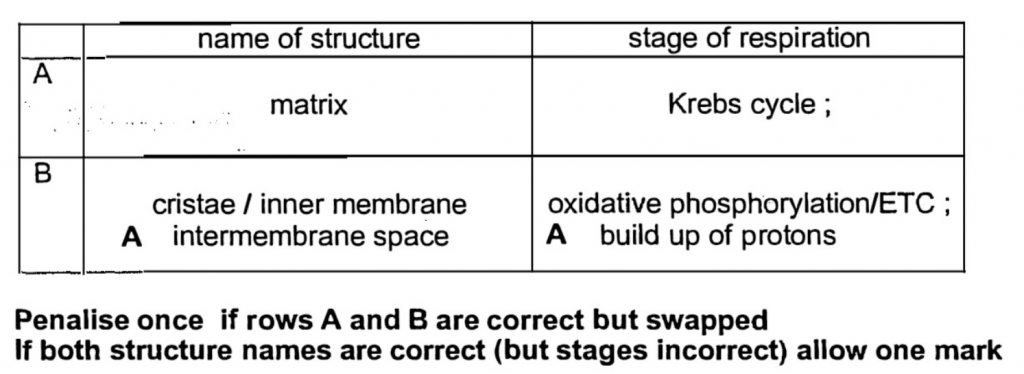
(b) Describe how the structure of a mitochondrion is adapted to carry out these two processes. [3]

(c) Describe briefly the role of NAD in respiration. [3]

(d) Describe how photophosphorylation differs from oxidative phosphorylation. [3]

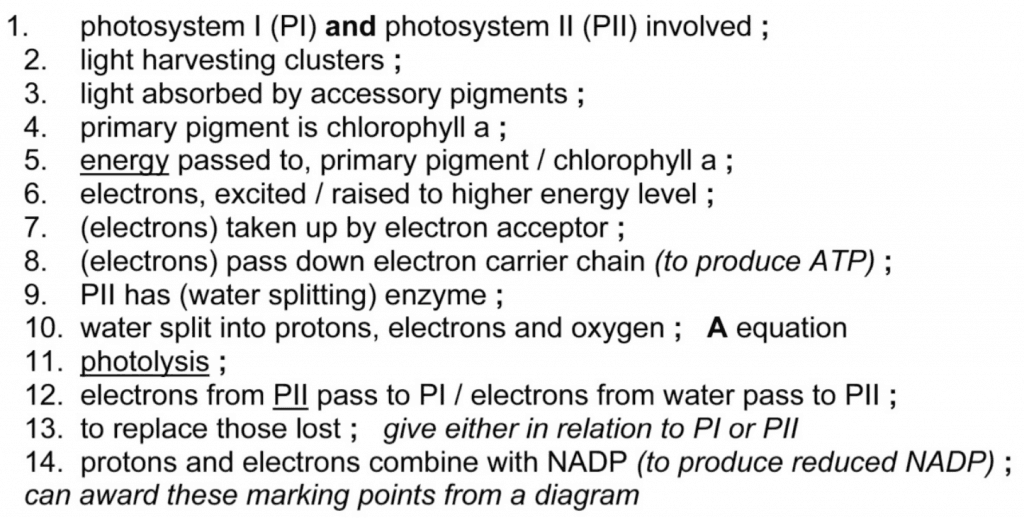
Describe how non-cyclic photophosphorylation produces ATP and reduced NADP. [9]
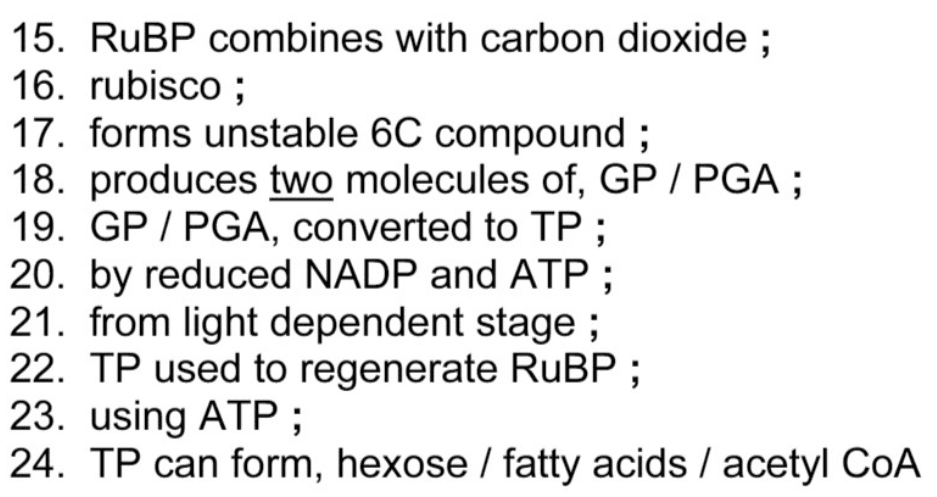
Outline the steps of the Calvin cycle. [6]
The initial stages of respiration convert one molecule of glucose into two molecules of a 3C compound.
State:
(i) the name given to these initial stages. [1]
(ii) where these stages occur in cells. [1]
(iii) the total number of ATP molecules formed during these stages. [1]

Most of the ATP formed in respiration is produced within the mitochondria by oxidative phosphorylation.
State the location, in the mitochondrion, of oxidative phosphorylation. [1]

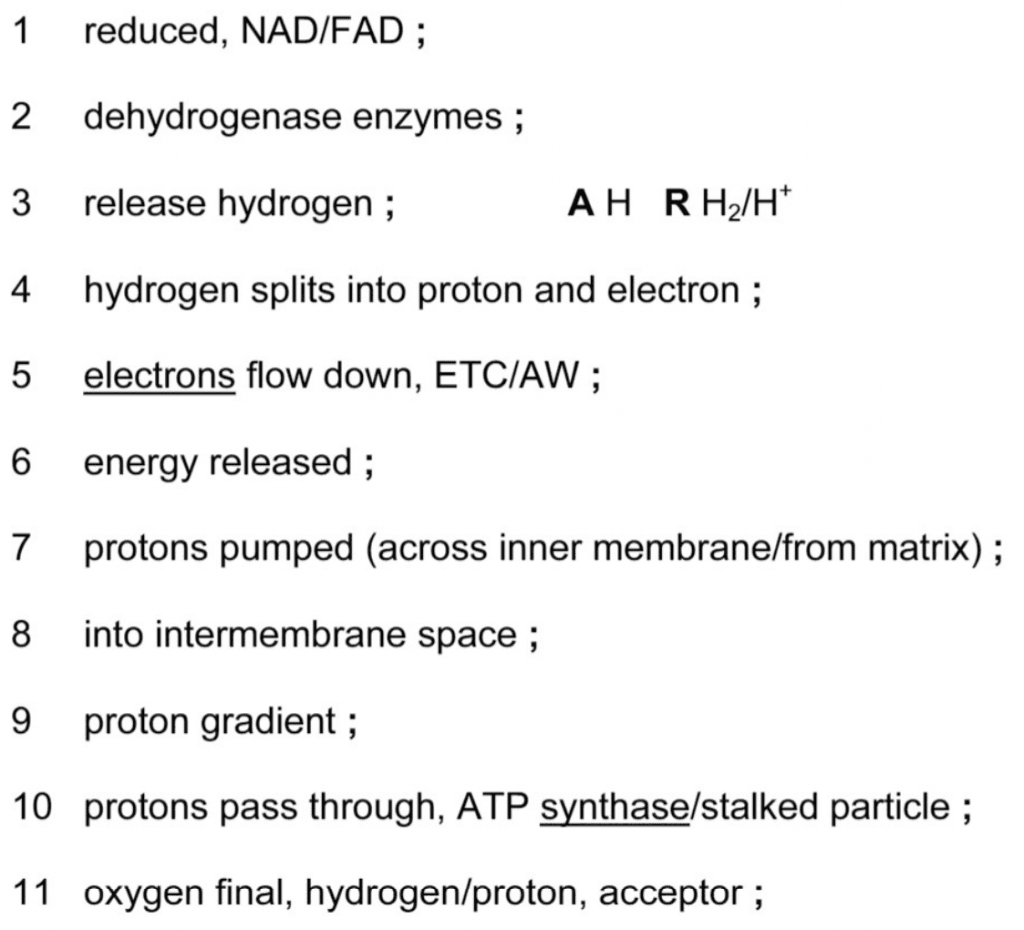
Outline the process of oxidative phosphorylation. [5]
In an investigation, mammalian liver cells were homogenised (broken up) and the resulting homogenate centrifuged. Samples of the complete homogenate and samples containing only nuclei, only ribosomes, only mitochondria or only the remaining cytosol
were incubated with:
1. glucose
2. pyruvate
3. glucose and cyanide
4. pyruvate and cyanide
Cyanide inhibits oxidative phosphorylation. After incubation the presence or absence of carbon dioxide and lactate in each sample was determined. The results are summarised in Table 7.1.
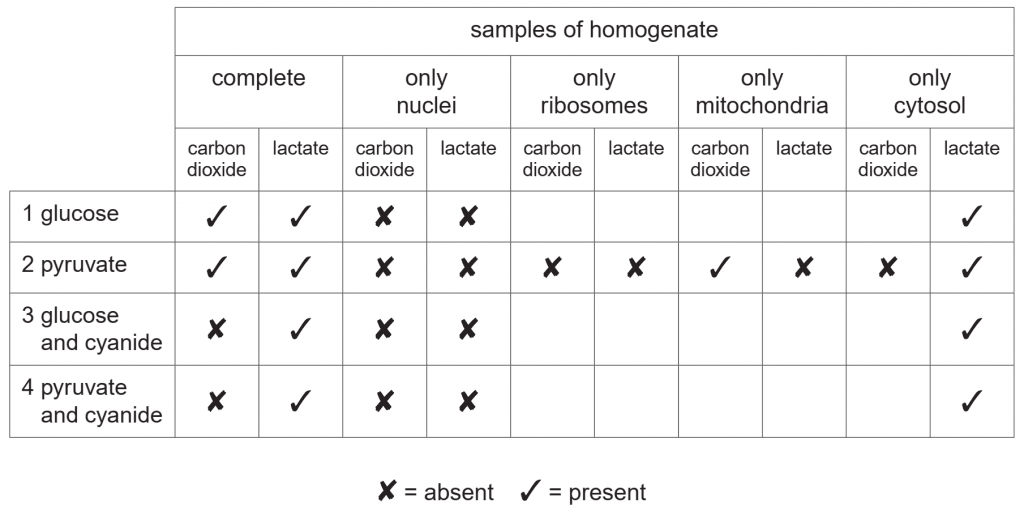
With reference to Table 7.1, name the two organelles not involved in respiration. [1]

Explain why carbon dioxide is produced when mitochondria are incubated with pyruvate but not when they are incubated with glucose. [3]

Explain why, in the presence of cyanide, lactate is produced but carbon dioxide is not. [3]

Question 3:


Question 4:


Question 5:
The diagram below shows the structure of ATP.
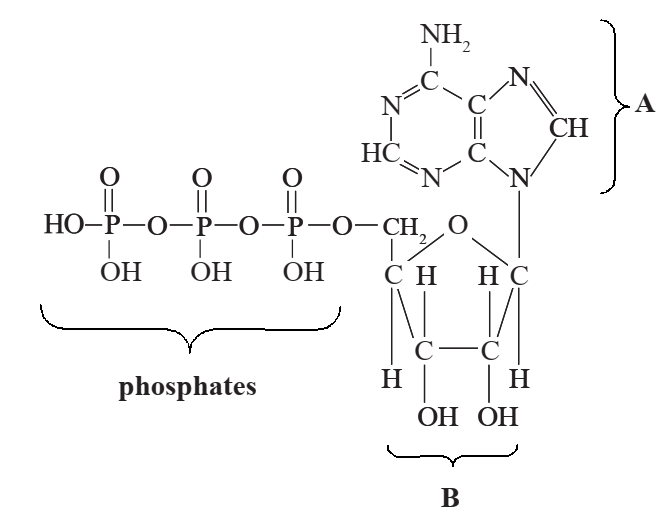
(a) Name each of the components:
(i) A: [1]
(ii) B: [1]
(i) adenine;
(ii) ribose;
(b)The table shows the relative energy levels of common phosphate-containing metabolites
(i) Suggest why ADP and ATP are effective energy carrier molecules. [2]
intermediate position;
means that they can accept or donate (energy rich) phosphate;
(ii) Suggest why high levels of creatine phosphate are found in striated muscle tissues. [2]
Any two of:
(very) high energy content/
allows rapid/sudden contraction/
since ATP synthesis is relatively slow;;
Question 6:
The figure shows the energy changes which occur during one stage of cellular respiration. A, B, C and D are intermediate compounds.

(a) State where in the cell this stage would take place. [1]
cytoplasm;
(b)Suggest an explanation for the energy changes between,
(i) Time 0 to T1: [2]
glucose activated by addition of phosphate/by ATP;
therefore intermediate B is at a higher energy level;
(ii) Time T1 to T2: [2]
energy taken from B and C;
trapped on ATP/NADH;
(c) In aerobic conditions what will be the immediate fate of the pyruvate? [2]
enters mitochondrion;
converted to acetyl coenzyme A;
(d) Outline the role of coenzymes in aerobic respiration. [3]
Any three of:
NAD/FAD/
receive electrons / H+/ are reduced/
coenzymes are reoxidised in the electron transport chain/
generating ATP/
by oxidative phosphorylation;;;
Question 7:
ATP is produced in both chloroplasts and mitochondria.
The table below compares the process of ATP production in these organelles. Complete the table with a tick (✓) in the appropriate box if the statement is true for ATP production in each organelle and a cross (×) if the statement is incorrect. [5]
| Statement | Chloroplast | Mitochondrion |
| Electrons are excited by photons | ||
| Electrons pass through carriers | ||
| Involves oxidative photophosphorylation | ||
| ATP produced from ADP and phosphate | ||
| Occurs in day and night |
| Statement: | ATP production in | ATP production in |
| Mitochondrion: | Chloroplast: | |
| Electrons are excited by photons | ✗ | ✓ |
| Electrons pass through carriers | ✓ | ✓ |
| Involves oxidative photophosphorylation | ✗ | ✓ |
| ATP produced from ADP and phosphate | ✓ | ✓ |
| Occurs in day and night | ✓ | ✗ |
Question 8:
Cyanide is a metabolic poison. Cyanide ions (CN–) bind to cytochrome oxidase which is the final carrier in the electron transport chain. This is shown in the figure below.

(a) Name substances X and Y. [2]
X pyruvate/acetyl coenzyme A;
Y CO2;
(b)State precisely where in the cell:
(i) The Krebs cycle occurs. [1]
matrix of mitochondrion;
(ii) electron carriers are situated. [1]
cristae/inner membrane of mitochondria;
(c) Explain the term ‘coupled redox reaction’. [3]
carriers are alternately reduced and oxidised;
gain of electrons/hydrogen = reduction/loss of electrons/hydrogen = oxidation;
linked to ATP synthesis/oxidative phosphorylation;
(d)Suggest why cyanide poisoning victims suffer from severe ATP shortage. [2]
cyanide stops the flow of electrons/blocks the electron transport chain/blocks cytochrome oxidase;
prevents regeneration of NAD/FAD from NADH/FADH or prevents reoxidation of NADH/FADH/cytochromes;
thus ATP synthesis is inhibited;
Question 9:
The diagram shows the sequence of events involved in glycolysis and Krebs cycle.

(a) Name the substances X and Y. [2]
X ATP;
Y CO2/hydrogen/H;
(b)State where in the cell the processes of glycolysis and Krebs cycle occur. [2]
glycolysis:
Krebs cycle:
glycolysis in cytoplasm;
Krebs in matrix of mitochondria;
(c) Use the information in the diagram to explain the term ‘feedback inhibition’. [3]
high levels of ATP;
inhibit conversion of intermediate 1 to intermediate 2;
prevents excess production of ATP;
Question 10:
The equation shows some of the stages in the process of glycolysis.

(a) Where in the cell does glycolysis occur? [1]
cytoplasm;
(b) Explain why ATP molecules are used in the first stage of glycolysis. [1]
provides activation energy/makes glucose more reactive;
(c) What type of chemical reaction is involved in the conversion of triose phosphate to pyruvate? [1]
dehydrogenation/oxidation/redox reaction/(if say reduction must specify NAD → NADH);
(d) How is NADH reoxidised in anaerobic conditions in:
(i) animals? [1]
(ii) yeast ? [1]
(i) hydrogen from NADH used to reduce pyruvate to lactate;
(ii) hydrogen from NADH used to reduce pyruvate to ethanol;
(e) Outline the process of oxidative phosphorylation. [3]
hydrogen/electrons removed from substrate/intermediate/named intermediate;
reference to carriers/NAD/FAD;
passed to successively lower energy levels;
energy released used to convert ADP into ATP/ phosphorylate ADP;
Question 11:
The table below summarises the process of cellular respiration. Complete the table by filling in the gaps. [9]
| Stage | Site | Oxygen Needed? | What Happens? |
| Glycolysis | Glucose is converted to ……………………………………… Hydrogen is removed and is passed to the electron carriers. | ||
| Matrix of Mitochondria | Yes | Pyruvate enters mitochondrion, is decarboxylated, dehydrogenated and combines with coenzyme A to give acetyl coenzyme A. The hydrogen which is removed is passed to the electron carriers. | |
| A cyclical series of reactions during which hydrogen is passed to the electron carriers, carbon dioxide is removed and a starting reactant is regenerated. | |||
| Electron Transfer Chain | Yes | The hydrogen from the respiratory reactions is split to release electrons. These pass through carriers and generate ………………………………… The hydrogen reforms and is combined with oxygen to release water. |
| Stage: | Site: | Oxygen Needed? | What Happens? |
| Glycolysis | Cytoplasm; | No; | Glucose is converted to pyruvic acid; Hydrogen is removed and is passed to the electron carriers. |
| Link Reaction; | Matrix of Mitochondria | Yes | Pyruvate enters mitochondrion, is decarboxylated, dehydrogenated and combines with coenzyme A to give acetyl coenzyme A. The hydrogen which is removed is passed to the electron carriers. |
| Kreb’s Cycle; | Matrix of Mitochondria; | Yes; | A cyclical series of reactions during which hydrogen is passed to the electron carriers, carbon dioxide is removed and a starting reactant is regenerated. |
| Electron Transfer Class | Crista/Inner Membrane of Mitochondria; | Yes | The hydrogen from the respiratory reactions is split to release electrons. These pass through carriers and generate ATP;. The hydrogen reforms and is combined with oxygen to release water. |
Question 12:
Read the following passage and answer the questions which follow.
The TCA cycle is also known as the citric acid or Krebs cycle. Pyruvate, synthesised in glycolysis is converted into acetyl coenzyme A which enters the TCA cycle. The rate at which the TCA cycle proceeds is carefully regulated. If ATP begins to accumulate, the rate is slowed. One such mechanism involves the allosteric inhibition by ATP of the enzyme isocitrate dehydrogenase.
(a) Outline how pyruvate is produced in glycolysis. [3]
Any three of:
glucose phosphorylated/activated/
using ATP/
split into 3C/triose phosphate/
oxidation/dehydrogenation of trioses yields pyruvate;;;
(b) State where in the cell glycolysis and the Krebs cycle take place.
(i) glycolysis. [1]
(ii) Krebs cycle. [1]
(i) cytoplasm;
(ii) matrix of mitochondria;
(c) Explain how allosteric inhibition can be used to control the rate of the cycle. [3]
Any three of:
ATP binds to enzyme/isocitrate dehydrogenase/
at a site other than the active site/
this changes the shape of the enzyme/active site/
therefore substrate cannot attach and process slowed/
this happens when too much ATP has been made;;;
(d) Outline the fate of pyruvic acid in yeast under anaerobic conditions. [4]
Any three of:
pyruvate decarboxylated to ethanal/
producing carbon dioxide/
ethanal converted/reduced to ethanol/
enabling NADH to be oxidised to NAD;;;
Question 13:
Suggest explanations for the following:
(a) Ethanol accumulates in the roots of plants which have been overwatered. [3]
Any three of:
overwatering leads to anaerobic condition/
anaerobic conditions inhibit electron transport chain/
thus pyruvic acid/pyruvate has been converted into ethanol/
to enable NADH to be reoxidised to NAD;
(b)Krebs cycle reactions continue even if glycolysis stops. [2]
Any two of:
acetyl coenzyme A can be formed from other substrates/
from breakdown of fats/
from deamination of amino acids;;
(c) Lactic acid accumulates in the body of a person who exercises vigorously. [4]
Any four of:
oxygen supply to muscles is inadequate during severe exercise/
thus electron transport system inhibited/
thus NADH cannot be reoxidised/
pyruvic acid converted to lactic acid/
changing NADH back to NAD;;;;
Question 14:
The diagram below shows some of the stages in cellular respiration in humans.

(a) Identify the following components:
(i) A. [1]
(ii) B. [1]
(iii) C. [1]
(i) lactate/lactic acid;
(ii) carbon dioxide;
(iii) electrons/hydrogen;[reject H2]
(b) State the significance of the following components:
(i) phosphorylase enzymes. [1]
(ii) dehydrogenase enzymes. [1]
(i) adds phosphate to ADP to produce ATP;
(ii) remove hydrogen from substrates/oxidise substrates/pass hydrogen to acceptors;
(c) Briefly explain why anaerobic respiration produces much less ATP than aerobic respiration. [4]
without oxygen there is no final acceptor for electrons/hydrogen from electron transport chain;
hence no regeneration /reoxidation of coenzymes;
thus Krebs cycle stops;
electron transport chain and Krebs cycle provide most of the ATP;
Question 15:
Individuals who become deficient in the vitamin B complex may suffer from a lack of energy. The table outlines
the functions of some of the vitamin B complex.
| Vitamin: | Function: |
| Niacin (Nicotinic acid) | Part of the NAD and NADP coenzymes |
| Riboflavin (B2) | Part of the prosthetic group FAD |
| Pantothenic acid | Part of coenzyme A |
(a) (i) What is meant by the term ‘coenzyme’? [2]
organic molecules necessary for enzyme function;
not permanently attached to the enzyme;
involved in transfer of hydrogen/electrons/acetate groups/energy/any other correct example;
(ii) What is meant by the term ‘prosthetic group’? [1]
similar to coenzyme but tightly bound to one specific enzyme;
(b)Suggest why individuals who are deficient in B complex vitamins may suffer from a lack of energy. [4]
vitamin B complex/nicotinic acid/riboflavin is required for synthesis of NAD/FAD;
pantothenic acid/coenzyme A required to produce acetyl CoA from pyruvate;
less acetyl CoA means less substrate for the Krebs cycle;
NAD/FAD are hydrogen acceptors in respiration;
if deficient electron transport chain may be impaired so less ATP produced;
Question 16:
The equation shows one part of the stages of respiration.

(a) Name this stage. [1]
glycolysis;
(b) Identify X and Y: [1]
X ATP;
Y ADP;
(c) State:
(i) the name of one process by which glucose enters cells. [1]
facilitated diffusion/active transport;
(ii) where in the cell this stage occurs. [1]
cytoplasm;
(d) Suggest:
(i) why glucose is first converted into glucose-6-phosphate. [1]
to make glucose reactive/phosphorylation gives energy of activation/keeps glucose inside cell/there are no
carriers for glucose-6-phosphate in the cell membrane/keeps concentration of free glucose inside cell low so
maintains concentration gradient;
(ii) why this stage is vital in red blood cells. [2]
they lack mitochondria;
therefore rely on glycolysis to provide energy;
Question 17:
The amino groups of unwanted amino acids are toxic to the body and so these amino groups are removed from
the amino acids as ammonia.
(a) (i) Where does this process mainly occur in the body? [1]
liver;
(ii) What is the name given to the process of forming ammonia from amino acids? [1]
deamination/transdeamination;
(iii) Explain why mammals do not excrete the ammonia directly as their main nitrogenous end product. [2]
limited solubility of ammonia in water means too much water would be lost when excreting the amounts of ammonia produced;
mammals being land animals cannot risk losing too much water;
The diagram below shows the Ornithine Cycle which is responsible for making urea from ammonia.

(b)(i) In which organ of the body does this process mainly occur? [1]
liver;
(ii) Where does the CO2 come from in step 1? [1]
(by-product of) respiration;
(iii) Why is the amino acid ornithine referred to as a “carrier substance”? [2]
because ammonia and carbon dioxide are attached to it to assemble urea;
it is reformed when the urea is split off it:
(iv) What type of reaction is step 3? [1]
hydrolysis;
(v) Why is urea a more suitable excretory end product than ammonia, in mammals? [2]
urea is more soluble in water than ammonia;
thus involves less water loss in urine which is advantageous to a land animal/no need to carry large volumes of urine around;
Question 18:
When substances cross biological membranes energy is either released or used.
(a) Distinguish between membrane transport by diffusion and membrane transport by active transport. [4]
diffusion is the movement of molecules down a concentration gradient;
energy is released as molecules diffuse;
active transport is the movement against a concentration gradient;
involves the expenditure of energy/ATP;
involves the use of carriers;
Chemiosmosis is the process in which energy released when a substance moves along a gradient is used to
synthesise ATP. The diagram below illustrates this mechanism.

(b)(i) What name is given to the chemical reaction that synthesizes ATP? [1]
oxidative phosphorylation;
(ii) What type of pumps are the electron chain carriers? Explain why. [2]
proton pumps;
because they move hydrogen ions which are protons;
(iii) What are the functions of ATPase? [3]
enables protons/hydrogen ions to diffuse back across the membrane;
ref to proton motive force/surplus of positive ions on inside of membrane;
movement of protons back releases energy;
which is harnessed by enzyme to convert ADP + P to ATP;
Question 19:
The respiratory quotient can yield information about the nature of the substrate being used for respiration and the type of metabolism that is occuring.
(a) Define the term ‘respiratory quotient’. [2]
the volume of carbon dioxide evolved;
divided by the volume of oxygen absorbed;
(or allow, moles/molecules of carbon dioxide evolved;
divided by moles/molecules of oxygen used;)
The following equation shows the aerobic respiration of the oil triolein(olive oil)

(b)(i) Calculate the respiratory quotient for the respiration of triolein. Show your working. [2]
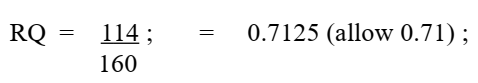
(ii) When the respiratory quotient of a human is measured the value is usually between 0.7 and 1.0. Explain
why this is so. [3]
RQ when respiring only carbohydrate would be 1.0;
because volume CO2 released (6CO2) equals volume of O2 used (6O2);
RQ when respiring only fat is 0.7;
humans respire both carbohydrate and fat at the same time;
The apparatus below can be used to demonstrate whether germinating peas respire purely carbohydrate or a mixture of carbohydrate and fat.

(c) (i) What would happen if the seeds were respiring: [2]
carbohydrate only.
carbohydrate and fat?
there would be no movement of the fluid in the manometer;
fluid in the manometer would move towards the peas;
(ii) Describe how you would use the apparatus to determine which substrates the germinating peas were using. [3]
use water bath/incubator to keep temperature constant thus avoiding gas volume changes;
stated suitable temperature (15 – 25oC);
have tap open and allow time (at least 10 minutes) to equilibrate;
close tap and allow to work for suitable time (at least 30 minutes);
(iii) If the peas were only respiring carbohydrates which metabolic processes would they be using? [1]
glycolysis, Krebs cycle, respiratory chain/electron transport chain;
Question 20:
Study the flow diagram below which refers to various metabolic pathways, and then answer the questions.
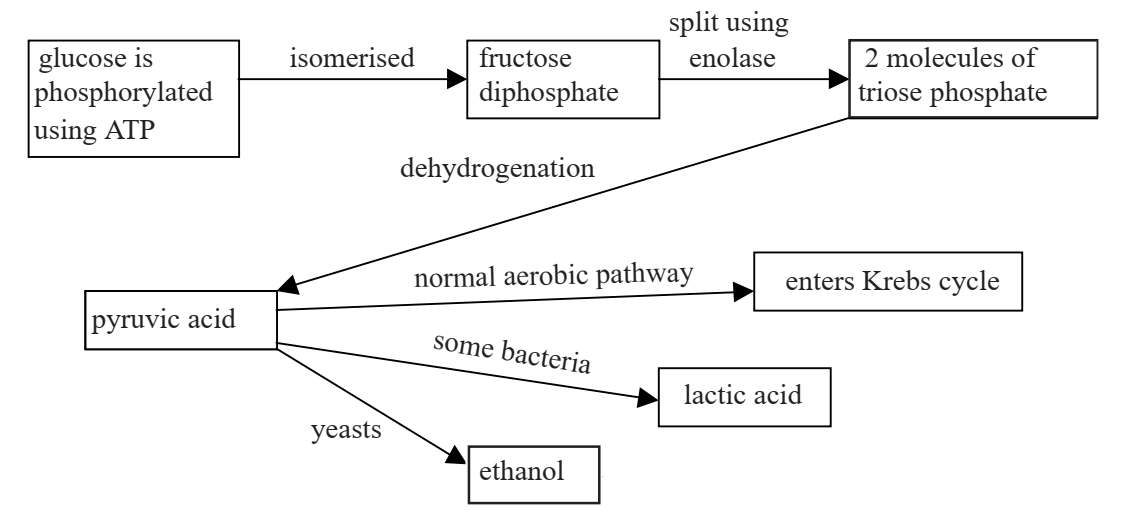
(a) (i) Why is it necessary to phosphorylate glucose using ATP? [2]
glucose must be activated/given energy before it can be metabolised/made more reactive;
phosphorylating with the energy rich bond of ATP gives glucose the extra energy needed;
(ii) What is isomerisation? [1]
changing to a different structural molecular shape whilst retaining the same empirical/molecular formula;
(iii) Suggest a reason for the isomerisation of glucose to fructose. [1]
enolase is substrate specific for fructose/cannot accept glucose as a substrate;
(iv) What is the importance of the dehydrogenation step in the pathway? [2]
to produce reduced NAD/NADH/NADH2;
which can be used/reoxidised in the respiratory chain/electron transport chain;
so that ATP is synthesized;
(b)(i) Explain why yeasts may sometimes respire to produce alcohol but at other times just produce carbon
dioxide and water. [4]
yeast respires/produces carbon dioxide and water, using the normal aerobic pathway when oxygen is available;
when oxygen is missing NADH2 has to be reoxidised without the use of the electron transport chain;
this is done by decarboxylating pyruvic acid/removing CO2 from pyruvic acid, to form ethanal;
this is hydrogenated/reduced to ethanol using NADH2 which is reoxidised to NAD;
(ii) Why do some bacteria, such as Lactobacilli produce lactic acid?. [2]
Lactobacilli are prokaryotic and so have no mitochondria/respiratory chain is not highly organised;
thus NADH2 is reoxidised by hydrogenating/reducing pyruvic acid to lactic acid;
(iii) State one commercial use of Lactobacilli. [1]
yoghurt manufacture;
(b)Fluoride is an inhibitor of the enzyme enolase. Why is it advantageous to add fluoride to toothpaste and drinking water? [2]
Lactobacilli/bacteria in the mouth/buccal cavity produce lactic acid;
this can cause decay/erode enamel of teeth;
if enolase is inhibited then the bacteria cannot produce the lactic acid;
(credit any reference to fluoride being incorporated into the enamel, thus hardening it)
Question 21:
The respiratory quotient (RQ) is useful since it will indicate the type of metabolism being carried out by an
organism. Carbohydrate respiration gives an RQ of 1.0, protein respiration an RQ of 0.9 and fat respiration an
RQ of 0.7.
(a) (i) What is meant by the term ‘respiratory quotient’? [2]
it is the volume of carbon dioxide /number of carbon dioxide molecules or moles liberated;
divided by the volume of oxygen/number of oxygen molecules or moles used;
(ii) Why do animals usually have an RQ between 0.7 and 1.0? [2]
because they do not respire only carbohydrate or only fat but a mixture of them;
protein is not usually respired in large amounts except in starvation;
(iii) When may organisms have an RQ less than 0.7? Give an example. [2]
when they are using the carbon dioxide for something else and so not releasing it;
for example, in photosynthesising plants;
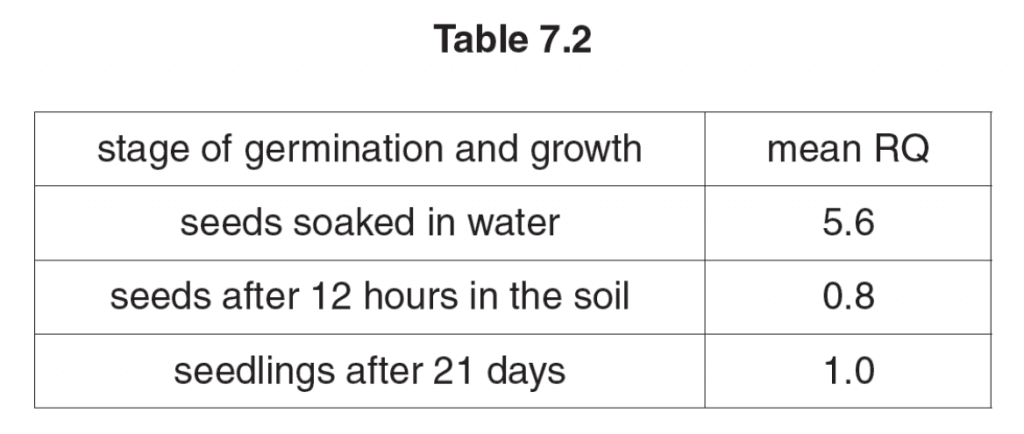
The graph below shows the RQs of barley seeds and castor oil seeds during germination.
(b)(i) Explain the different values for the RQs of barley and castor oil over the first 30 hours. [2]
barley seeds are respiring mainly starch and so have a RQ approaching 1.0;
castor oil seeds store oil and so are respiring fat (oil) and so have a RQ approaching 0.7;
barley seeds contain little lipid whereas castor oil seeds contain little starch;
(ii) Explain the changes in the RQ of the two sets of seeds after 40 hours. [2]
photosynthetic leaves/cotyledons/coleoptiles will have formed;
thus starch synthesis occurs and both sets of seeds can now respire carbohydrate;
initial starch content/oil content probably used up;
Question 22:
The diagram below shows stages in the deamination of the amino acid alanine.

(a) (i) Why is deamination necessary? [2]
amino acids cannot be stored since their amine groups are toxic;
deamination removes the amine groups of surplus amino acids for excretion;
(ii) Where does deamination occur in the human body? [1]
in the liver/hepatic cells;
(b)(i) Explain why deamination can be considered a respiratory process as well as an excretory process. [3]
it is respiratory since NADH is produced by dehydrogenation (and this can be used to generate ATP);
it also generates pyruvic acid which can be used in the Krebs cycle (to generate more ATP);
it is excretory since it removes toxic amine groups as ammonia (for excretion);
(ii) Explain what happens to the:
pyruvic acid at A: [2]
pyruvic acid forms acetyl coenzyme A in the link reaction;
which is further metabolised in the Krebs cycle;
NADH at B: [2]
NADH is reoxidised to NAD in the respiratory chain/electron transport chain;
resulting in ATP synthesis;
NH3 at C: [2]
ammonia enters the ornithine cycle to be assembled into urea;
which is transported by the blood to the kidneys for excretion;
Question 23:
Read through the following passage about cellular respiration and then complete it by writing the most appropriate
word or words in the spaces.
During glucose metabolism the glucose is first made metabolically mobile by ……………………………………….. using the energy rich substance …………………………………………… The mobilised glucose is then isomerised to make……………………………………………. which is then split with the enzyme enolase to yield two molecules of ………………………………………….. . This eventually undergoes dehydrogenation yielding the reduced coenzyme …………………………………… and pyruvic acid. This overall process is called ………………………………. ……and
occurs in the ……………………………… . The reduced coenzyme enters an organelle called a …………………………… and is reoxidised on the …………………………………………. membrane by the …………………………………….. to form …………………………………..molecules of ATP. Water is produced as a byproduct as the…………………………………. are removed by combination with ……………………………. under the influence of the enzyme ………………………. oxidase. The pyruvic acid is converted to acetyl-coenzyme A in the ……………………………. reaction and is then metabolised in the ……………………………… pathway.
phosphorylation/adding phosphate; ATP/adenosine triphosphate; fructose diphosphate; triose phosphate; NADH;
glycolysis; cytoplasm; mitochondrion; inner; respiratory chain/electron transport chain; three; protons/hydrogen ions; oxygen; cytochrome; link; Krebs cycle;
Question 24:
For each of the following statements say whether it is true or false and give an explanation for your answer.
(a) Proteins and amino acids are only respired in humans during times of extreme starvation. [5]
false;
body proteins are continually recycled/broken down and replaced;
amino acids formed are toxic;
so if not immediately used for protein reassembly must be deaminated;
surplus dietary amino acids are also deaminated;
deamination generates NADH (for ATP synthesis) and so is a respiratory process;
(b)The camel stores and respires fat rather than carbohydrate because it lives in a desert environment. [4]
true;
camel will be short of water (in desert);
water is a byproduct of respiration;
respiration of 1 gramme of fat yields more water than respiration of 1 gramme of carbohydrate;
almost twice as much water yielded from fat;
(c) Yeast cells always produce ethanol when respiring. [3]
false;
if oxygen is available yeast respires by the aerobic pathway;
when oxygen is not available NADH must be reoxidised in an alternative way;
by converting pyruvic acid to ethanol;