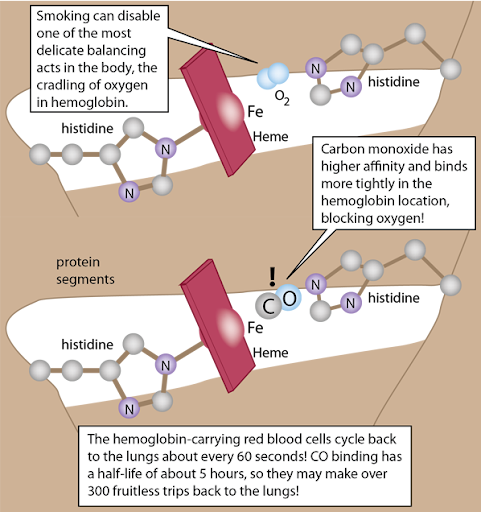
8.07 Gas Transport In The Blood
I. Oxygen Transport from Tissues to Blood
A. Oxygen Release from Hemoglobin in Tissues
- Low Oxygen Tension in Tissues:
- Partial Pressure: In actively respiring tissues, the partial pressure of oxygen (pO₂) is low due to high cellular consumption.
- Hemoglobin Affinity: Lower pO₂ and higher concentrations of CO₂ and H⁺ reduce hemoglobin’s affinity for O₂ (Bohr Effect).
- Oxygen Unbinding:
- Allosteric Changes: The Bohr Effect causes hemoglobin to undergo a conformational change, reducing its affinity for O₂.
- (Allosteric: refers to the regulation of a protein’s activity through the binding of a molecule (called an effector) at a site other than the protein’s active site. This binding site is known as the allosteric site, and the resulting changes in protein structure can either enhance or inhibit the protein’s function).
- Release of O₂: O₂ molecules dissociate from the heme groups of hemoglobin and diffuse into the surrounding tissue cells.
- Allosteric Changes: The Bohr Effect causes hemoglobin to undergo a conformational change, reducing its affinity for O₂.
B. Diffusion of Oxygen into Tissue Cells:
- Concentration Gradient:
- High to Low Gradient: O₂ diffuses from the blood (higher pO₂) into tissue cells (lower pO₂) following the concentration gradient.
- Cellular Utilization:
- Metabolic Use: Cells utilize O₂ for aerobic respiration, producing ATP, water, and CO₂ as byproducts.
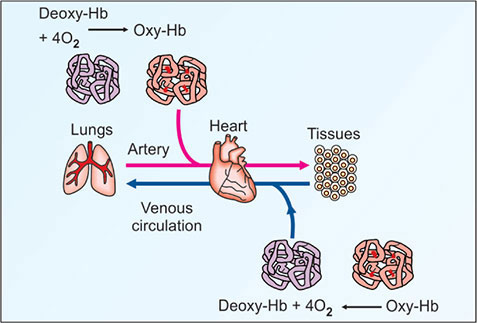
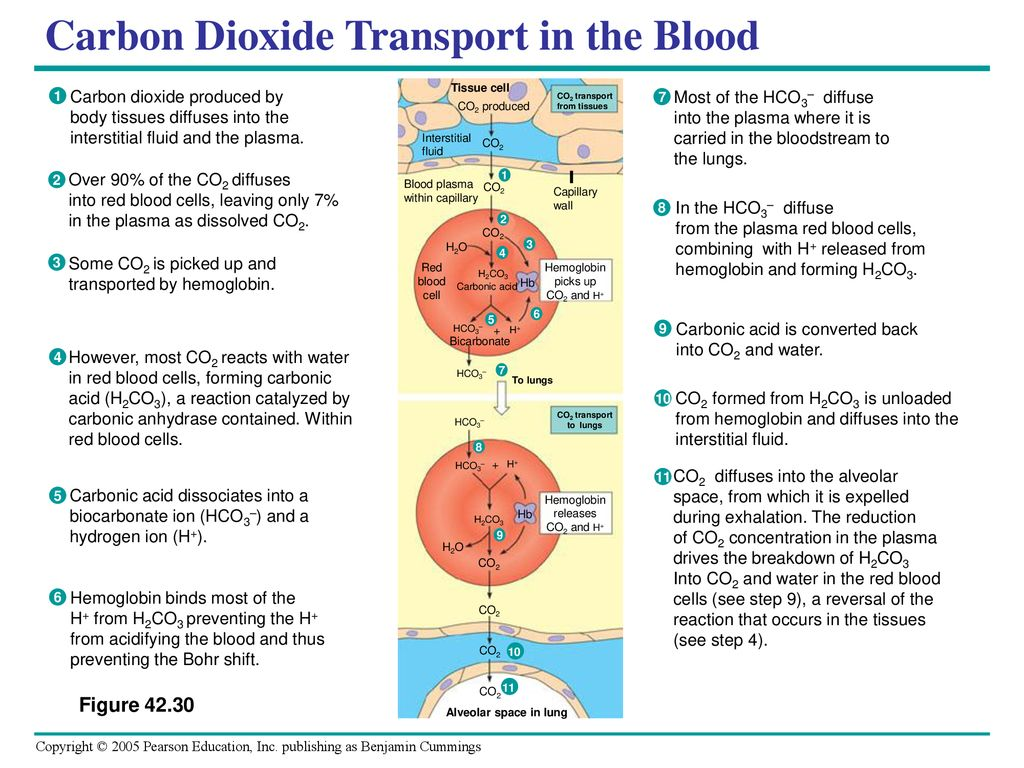
II. Carbon Dioxide Transport from Tissues to Blood
A. Production and Diffusion of CO₂ in Tissues
- Metabolic Byproduct:
- CO₂ Generation: Cellular metabolism produces CO₂, which diffuses out of cells into the interstitial fluid and then into capillary blood.
B. Entry of CO₂ into Red Blood Cells (RBCs)
- Diffusion into RBCs:
- Concentration Gradient: CO₂ diffuses from the interstitial fluid (high pCO₂) into RBCs (lower pCO₂).
- Conversion to Bicarbonate:
- Enzymatic Reaction: Inside RBCs, CO₂ reacts with water (H₂O) to form carbonic acid (H₂CO₃), catalyzed by carbonic anhydrase.
- Dissociation: H₂CO₃ dissociates into hydrogen ions (H⁺) and bicarbonate ions (HCO₃⁻).
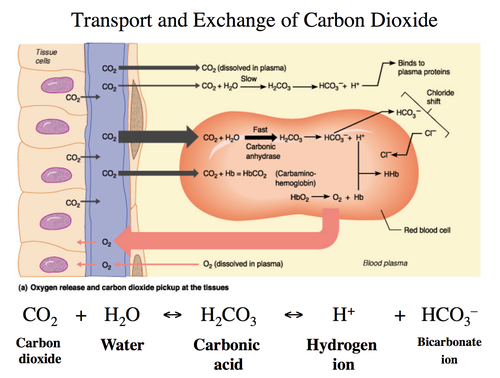
C. Transport of CO₂ in Blood
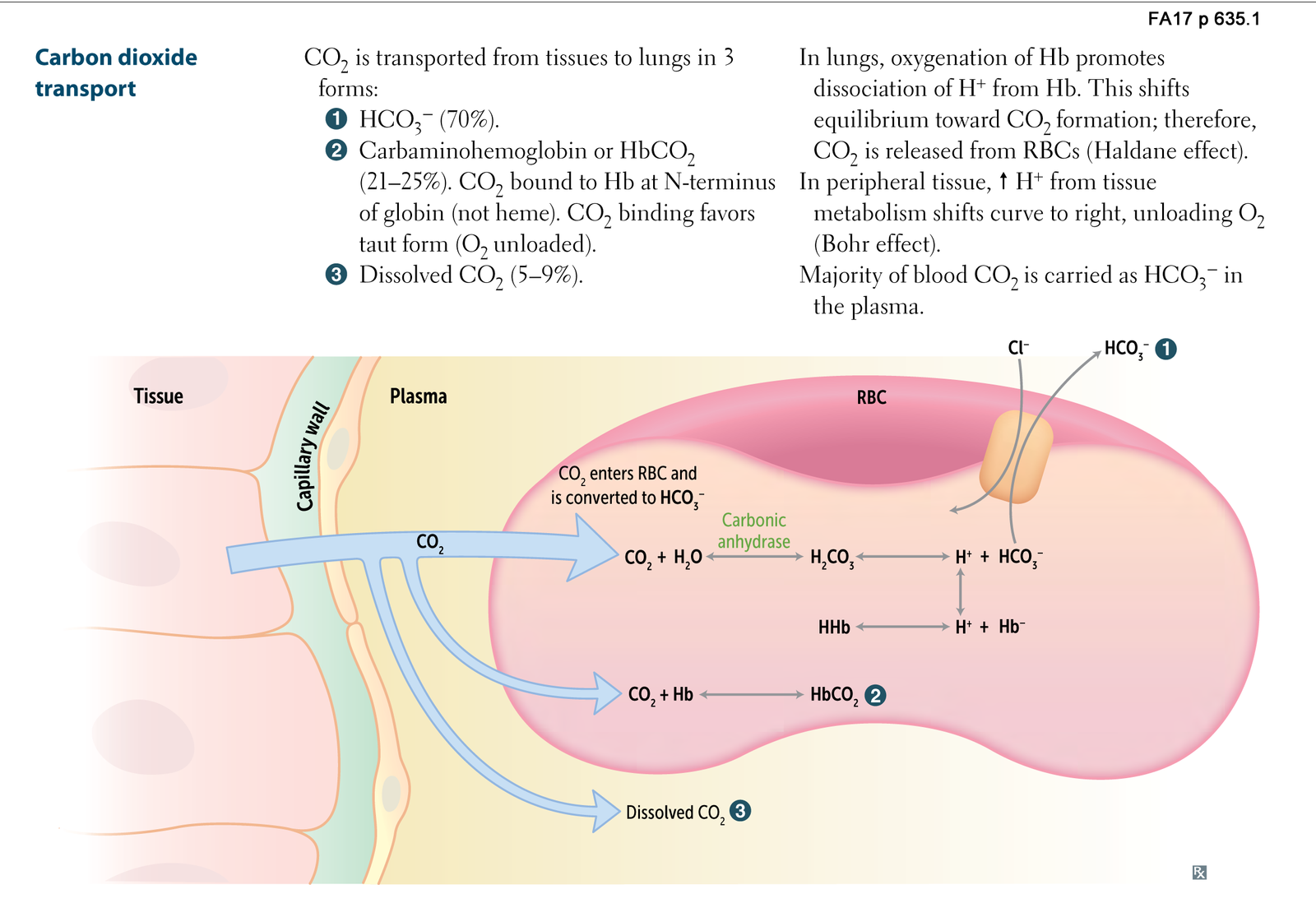
- Bicarbonate Transport (Major Pathway – 70-80%):
- Chloride Shift:
- The chloride shift occurs in red blood cells during gas exchange. In the tissues:
- Carbon dioxide (CO₂) diffuses into RBCs and reacts with water (H₂O) to form carbonic acid (H₂CO₃) via the enzyme carbonic anhydrase.
- Carbonic acid dissociates into HCO₃⁻ (bicarbonate) and H⁺ (hydrogen ion).
- The bicarbonate ion (HCO₃⁻) is then transported out of the RBC into the plasma.
- To maintain electrical neutrality, a chloride ion (Cl⁻) from the plasma enters the RBC to replace the lost negative charge. This is known as the chloride shift.
- The chloride shift occurs in red blood cells during gas exchange. In the tissues:
- Chloride Shift:
- Carbaminohemoglobin Formation (Minor Pathway – 10-20%):
- Binding to Hemoglobin: CO₂ binds to the amino (-NH₂) groups on the globin chains of hemoglobin, forming carbaminohemoglobin (HbCO₂).
- Dissolved CO₂ (Minor Pathway – 7-10%):
- Direct Transport: A small fraction of CO₂ remains dissolved in plasma, directly transported to the lungs.
III. Oxygen Transport from Blood to Lungs
A. Oxygen Uptake in the Lungs
- High Oxygen Tension in Lungs:
- Partial Pressure: In the alveoli, pO₂ is high, creating a concentration gradient favoring O₂ uptake into the blood.
- Diffusion into Blood:
- From Alveoli to Plasma: O₂ diffuses across the alveolar-capillary membrane into the plasma and then into RBCs.
- Binding to Hemoglobin:
- Oxyhemoglobin Formation: O₂ binds to the ferrous (Fe²⁺) heme groups of hemoglobin, forming oxyhemoglobin (HbO₂).
- Increased Affinity: The high pO₂ and lower concentrations of CO₂ and H⁺ in the lungs enhance hemoglobin’s affinity for O₂ (Haldane Effect).
B. Reversal of the Chloride Shift
- Bicarbonate Re-entry:
- Exchange Mechanism: Bicarbonate ions (HCO₃⁻) re-enter RBCs from plasma in exchange for chloride ions (Cl⁻) exiting RBCs.
- Conversion Back to CO₂:
- Chemical Reaction: Inside RBCs, bicarbonate combines with hydrogen ions (H⁺) to form carbonic acid (H₂CO₃), which is then converted back to CO₂ and H₂O by carbonic anhydrase.
- CO₂ Diffusion:
- Exhalation: CO₂ diffuses from RBCs into the alveoli to be exhaled.
IV. Carbon Dioxide Transport from Blood to Lungs
A. Release of CO₂ in the Lungs:
- Dissociation of Carbaminohemoglobin:
- Lower pCO₂: In the lungs, lower pCO₂ and higher pO₂ cause carbaminohemoglobin to release CO₂.
- Reconversion of Bicarbonate to CO₂:
- Enzymatic Reaction: Bicarbonate ions re-enter RBCs and combine with H⁺ to form carbonic acid, which is converted back to CO₂ and H₂O.
- Diffusion into Alveoli:
- From Blood to Alveoli: CO₂ diffuses from RBCs into the plasma, then across the alveolar-capillary membrane into the alveoli for exhalation.
V. Detailed Step-by-Step Flow
- To provide a comprehensive overview, here’s a sequential flow of events highlighting how O₂ and CO₂ are transported between tissues and lungs:
A. In Tissues (Peripheral Capillaries):
- a. Oxygen Unloading: – Low pO₂ and High pCO₂: Due to cellular respiration, tissues have low pO₂ and high pCO₂. – Bohr Effect: Elevated CO₂ and H⁺ reduce hemoglobin’s affinity for O₂. – O₂ Release: O₂ dissociates from oxyhemoglobin and diffuses into tissue cells.
- b. Carbon Dioxide Uptake: – CO₂ Production: Cells produce CO₂ as a metabolic waste product. – Diffusion into RBCs: CO₂ diffuses into RBCs from tissues.
- c. Conversion to Bicarbonate: – Carbonic Anhydrase Action: CO₂ + H₂O ⇌ H₂CO₃ ⇌ H⁺ + HCO₃⁻. – Bicarbonate Transport: HCO₃⁻ exits RBCs into plasma via the chloride shift.
- d. Carbaminohemoglobin Formation: – CO₂ Binding: Some CO₂ binds to hemoglobin, forming HbCO₂.
B. In Bloodstream (Systemic Circulation):
- a. Transport of Bicarbonate and Carbaminohemoglobin: – Bicarbonate Ions: Majority transported as HCO₃⁻ in plasma. – Carbaminohemoglobin: CO₂ carried bound to hemoglobin.
- b. Maintaining Electrochemical Balance: – Chloride Shift: Exchange of Cl⁻ and HCO₃⁻ maintains ionic balance.
C. In Lungs (Pulmonary Capillaries):
- a. Oxygen Uptake: – High pO₂ Environment: O₂ diffuses from alveoli into blood. – Binding to Hemoglobin: O₂ binds to hemoglobin, forming oxyhemoglobin (HbO₂).
- b. CO₂ Release: – Lower pCO₂ Environment: HbCO₂ dissociates, releasing CO₂. – Bicarbonate Reversion: HCO₃⁻ re-enters RBCs and converts back to CO₂.
- c. Chloride Shift Reversal: – Exchange Mechanism: Cl⁻ exits RBCs as HCO₃⁻ re-enters, restoring ionic balance.
- d. Exhalation of CO₂: – Diffusion into Alveoli: Released CO₂ diffuses into alveoli to be exhaled.
C. Exhalation:
- Removal of CO₂: CO₂ is expelled from the body during exhalation, completing the gas exchange cycle.
VI. Illustrative Flowchart
To visualize the process, consider the following flowchart:
- Tissues:
- Low pO₂, High pCO₂ → O₂ released from Hb → O₂ diffuses into cells.
- CO₂ produced → CO₂ diffuses into RBCs → CO₂ converted to HCO₃⁻ + H⁺.
- Bloodstream:
- HCO₃⁻ transported in plasma → Cl⁻ enters RBCs (Chloride Shift).
- Some CO₂ bound to Hb as HbCO₂.
- Lungs:
- High pO₂, Low pCO₂ → O₂ binds to Hb → HbO₂ formed.
- HbCO₂ releases CO₂ → HCO₃⁻ re-enters RBCs + H⁺ combines to form H₂CO₃ → CO₂ released.
- Cl⁻ exits RBCs as HCO₃⁻ re-enters.
- Exhalation:
- CO₂ expelled from alveoli → Removed from body.
VII. Key Mechanisms Facilitating Gas Exchange
Bohr Effect:
- Function: Lowers hemoglobin’s affinity for O₂ in the presence of high CO₂ and H⁺, enhancing O₂ release in tissues.
- Location: Predominantly in peripheral tissues.
Haldane Effect:
- Function: Enhances CO₂ release in the lungs by increasing hemoglobin’s affinity for O₂, promoting O₂ binding and CO₂ dissociation.
- Location: Predominantly in the lungs.
Chloride Shift (Hamburger Phenomenon):
- Function: Maintains electrochemical neutrality by exchanging HCO₃⁻ and Cl⁻ across the RBC membrane.
- Significance: Facilitates efficient transport of CO₂ in the form of bicarbonate.
Carbonic Anhydrase Activity:
- Function: Catalyzes the reversible reaction between CO₂ and H₂O to form H₂CO₃, speeding up the conversion to H⁺ and HCO₃⁻.
- Location: Abundant in RBCs.