11.03 Phagocytes
Phagocytes
Function
- Engulf and Destroy Pathogens: Phagocytes ingest harmful microorganisms and particles, neutralizing them.
- Remove Dead Cells: They clear cellular debris and apoptotic cells, maintaining tissue health and homeostasis.

Types of Phagocytes
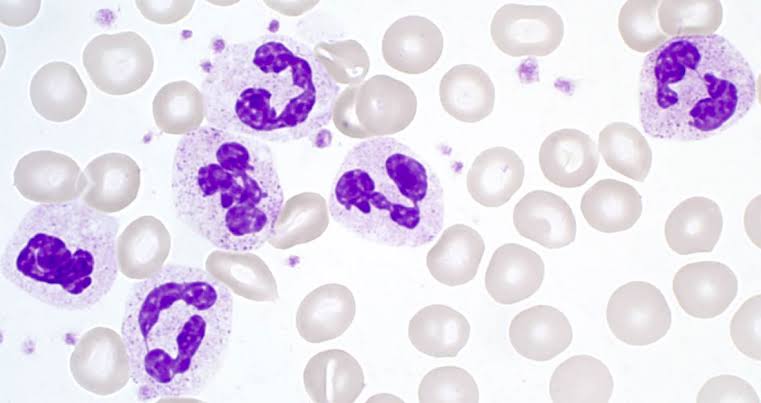
1. Neutrophils
- Prevalence: Constitute approximately 60% of white blood cells.
- Lifespan: Short-lived; their numbers rapidly increase during infections.
- Migration: Travel through blood and capillaries to reach sites of infection.
- Mechanism: Utilize phagocytosis to engulf pathogens and subsequently die, contributing to pus formation.
2. Macrophages
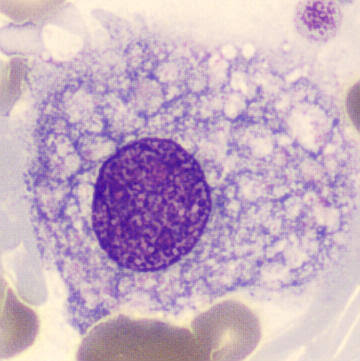
- Size & Lifespan: Larger and more long-lived compared to neutrophils.
- Location: Predominantly found in organs such as the lungs, liver, and spleen.
- Origin: Develop from monocytes in the blood, which differentiate into macrophages within tissues.
- Immune Activation: Present pathogen antigens on their surface to activate lymphocytes, bridging innate and adaptive immunity.
Observations
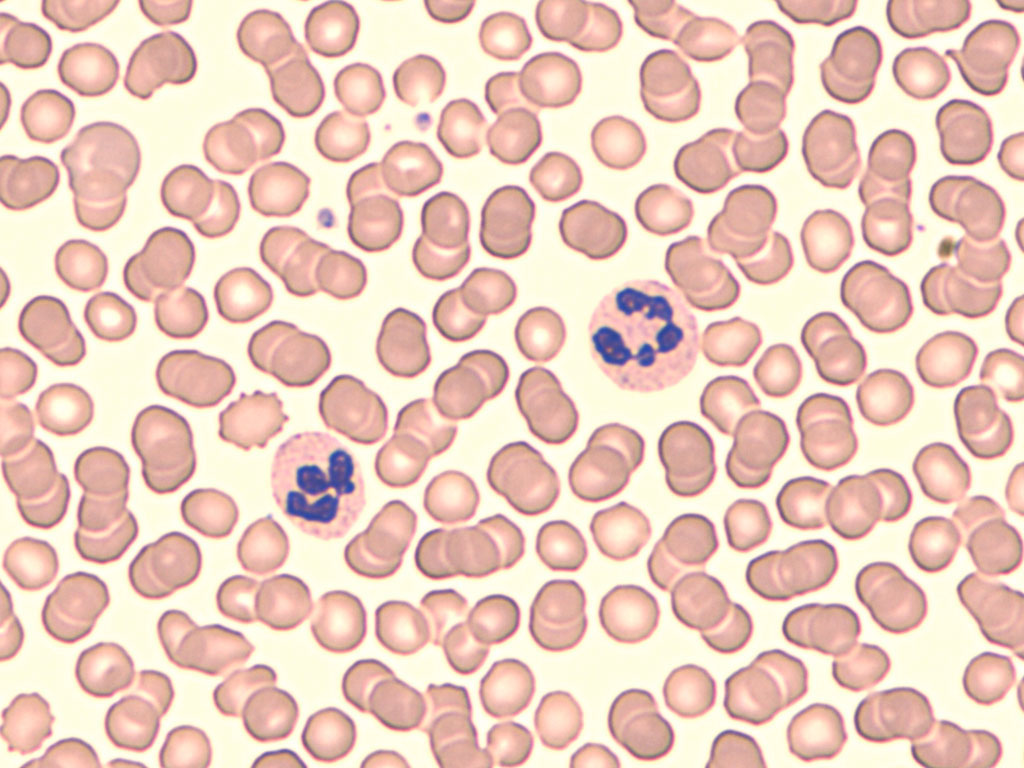
- Figure A: Blood smear under light microscope; displays monocyte (precursor to macrophage), neutrophil, and lymphocyte among red blood cells.
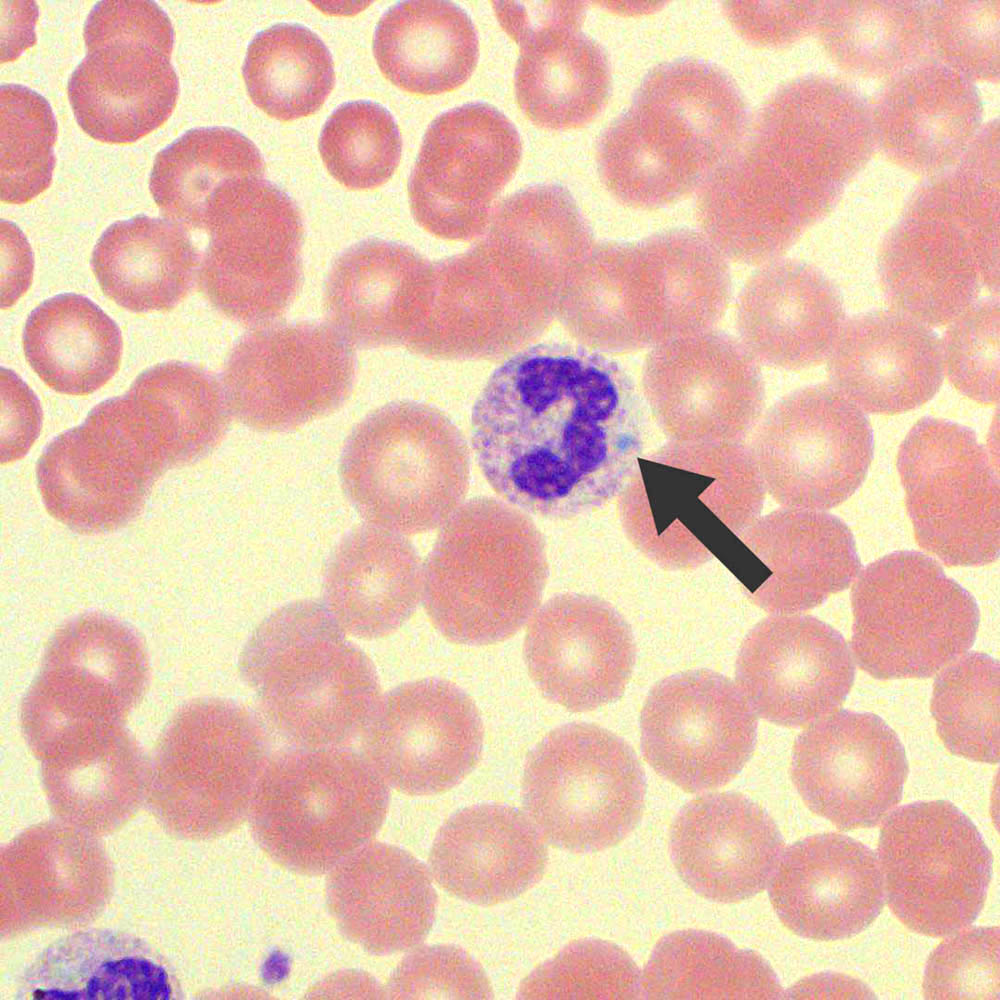
- Figure B: Showing neutrophil cell.
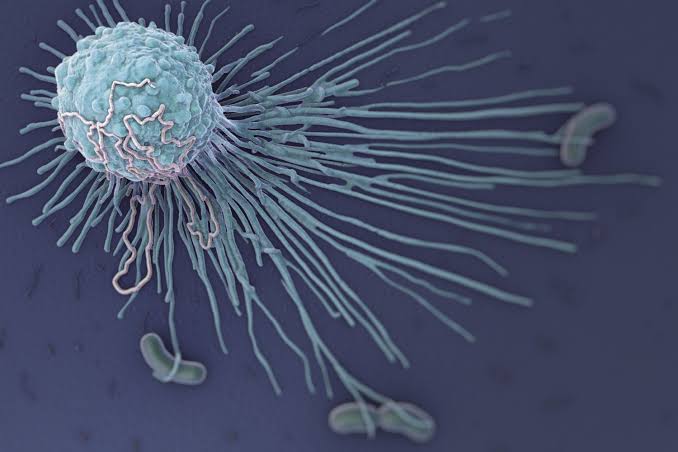
Phagocytosis Process
- Phagocytosis is a multi-step process through which phagocytes eliminate pathogens and debris.
1. Initiation of Phagocytosis
- Phagocytosis can be initiated through multiple pathways, ensuring flexibility and efficiency in the immune response.
a. Chemotaxis (Primary Initiator)
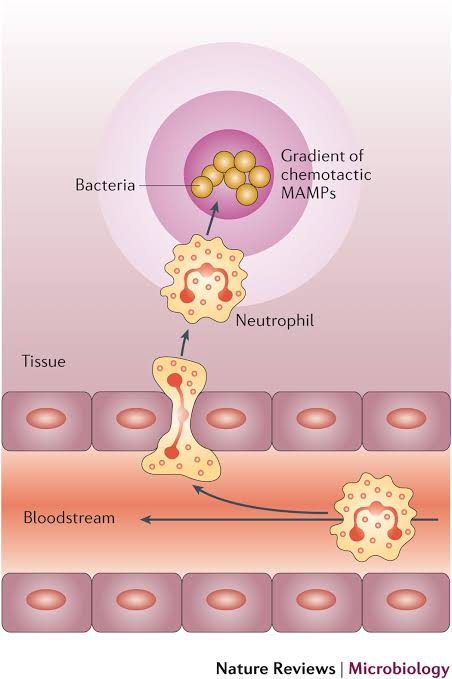
- Definition: Directed movement of phagocytes toward higher concentrations of specific chemical signals at the infection site.
- Mechanism:
- Chemical Signals Involved:
- Complement Proteins: Part of the complement system that marks pathogens and attracts phagocytes.
- Cytokines (e.g., Interleukins): Signaling molecules released by various cells to mediate and regulate immunity.
- Bacterial Products: Substances released by bacteria that attract phagocytes.
- Histamine and Other Inflammatory Mediators: Released during inflammation to increase blood flow and attract immune cells.
- Chemical Signals Involved:
- Outcome: Phagocytes migrate from the bloodstream through capillary walls to the affected tissue area.
b. Resident Phagocytes (Chemotaxis-Independent Initiation)
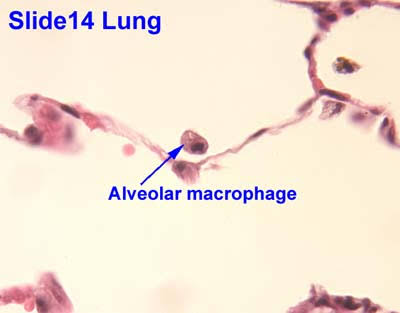
- Definition: Phagocytes that reside permanently within tissues (e.g., macrophages in the liver, lungs [alveolar macrophages], and brain [microglia]).
- Mechanism:
- Local Encounter: These phagocytes are already present within tissues and can immediately interact with pathogens or debris entering their local environment.
- Minimal Migration Needed: Only slight movement within the tissue is required to encounter and engulf targets.
- Example: Alveolar macrophages in the lungs swiftly respond to inhaled pathogens without needing to migrate extensively.
c. Direct Recognition and Engagement
- Definition: Phagocytes recognize and bind to pathogens directly through pattern recognition receptors without the need for opsonins (antibodies or complement proteins).
- Role: Allows phagocytes to initiate engulfment upon encountering pathogens in their immediate vicinity, independent of chemotactic signals.
d. Contact-Mediated Phagocytosis
- Definition: Activation of phagocytes through direct cell-to-cell contact with infected or abnormal cells.
- Mechanism:
- Recognition of “Eat Me” Signals: Infected or apoptotic cells display specific signals (e.g., phosphatidylserine) recognized by phagocytes.
- Engulfment Trigger: These signals prompt phagocytes to engulf and remove the targeted cells without the need for chemotactic movement.
- Example: Macrophages removing apoptotic cells during tissue remodeling and repair.
2. Recognition & Attachment
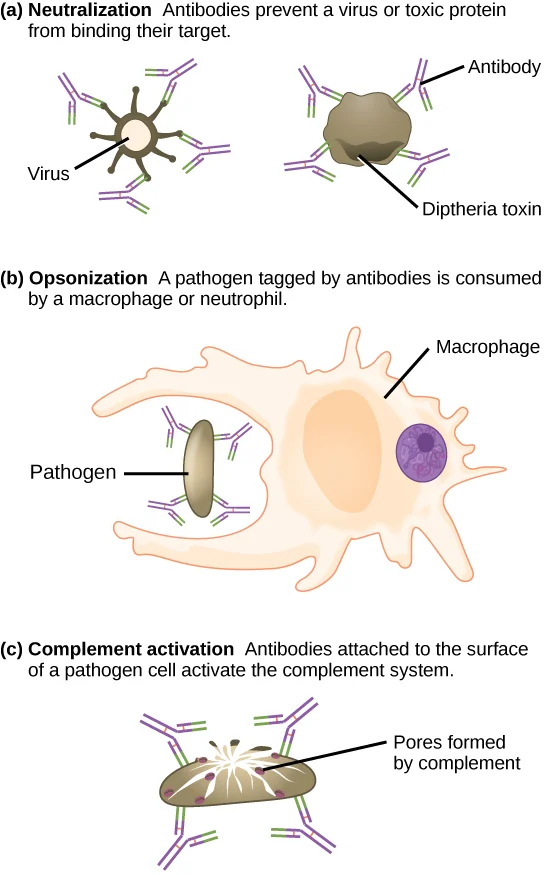
- Pathogen Marking (Opsonization): Pathogens are often coated with antibodies (IgG) or complement proteins, enhancing their recognition.
- Receptor Binding: Phagocytes possess specific receptors that bind to these markers.
- Specificity: This receptor-mediated binding ensures that phagocytes selectively target pathogens while sparing host cells.
3. Engulfment (Endocytosis)
- Membrane Extension: The phagocyte’s plasma membrane extends around the pathogen.
- Phagocytic Vacuole Formation: The membrane fully engulfs the pathogen, enclosing it within a vesicle known as a phagosome.
- Internalization: The phagosome is internalized into the phagocyte’s cytoplasm.
4. Lysosome Fusion
- Phagolysosome Formation: The phagosome fuses with a lysosome, an organelle containing digestive enzymes.
- Enzyme Release: Lysosomal enzymes (e.g., proteases, lipases) are released into the phagosome.
- Pathogen Degradation: The hostile environment within the phagolysosome breaks down the pathogen’s structural components.
5. Digestion & Exocytosis
- Degradation: Enzymes digest the pathogen into smaller, harmless molecules.
- Residual Body Formation: Indigestible material remains as a residual body within the phagocyte.
- Exocytosis: Waste products are expelled from the phagocyte by merging the residual body with the plasma membrane, releasing the debris outside the cell.
Key Terms
- Chemotaxis: The movement of cells toward higher concentrations of specific chemicals, typically at sites of infection or inflammation.
- Opsonization: The process by which pathogens are marked for ingestion and destruction by phagocytes through the coating of antibodies or complement proteins.
- Endocytosis: The cellular process of engulfing external substances by enveloping them in a vesicle.
- Lysosome: An organelle containing hydrolytic enzymes used to break down ingested pathogens and debris.
- Phagolysosome: A vesicle formed by the fusion of a phagosome with a lysosome, where digestion of the engulfed material occurs.
- Reactive Oxygen Species (ROS): Highly reactive molecules used by phagocytes to destroy pathogens.
- Pattern Recognition Receptors (PRRs): Receptors on phagocytes that recognize pathogen-associated molecular patterns (PAMPs).
- Phagocytic Vacuole (Phagosome): A vesicle formed around a pathogen when it is engulfed by a phagocyte.
- Residual Body: Indigestible material remaining within a phagocyte after digestion of a pathogen.