15.10 Chapter Summary
BioCast:
1. Features of the Endocrine System: ADH, Glucagon, and Insulin
Endocrine System Overview:
- The endocrine system consists of glands that secrete hormones directly into the bloodstream.
- Hormones act as chemical messengers regulating various body functions, including metabolism, growth, and homeostasis.
Key Hormones:
a. Antidiuretic Hormone (ADH):
- Produced by: Posterior pituitary gland.
- Function: Regulates water balance in the body by increasing water reabsorption in the kidneys’ nephrons, reducing urine volume.
- Mechanism: ADH binds to receptors in the collecting ducts, triggering the insertion of aquaporin channels, allowing water to be reabsorbed.
b. Glucagon:
- Produced by: Alpha cells of the pancreas (islets of Langerhans).
- Function: Raises blood glucose levels by promoting glycogenolysis (breakdown of glycogen to glucose) and gluconeogenesis (formation of glucose from non-carbohydrate sources) in the liver.
- Mechanism: Glucagon binds to receptors on liver cells, activating cAMP pathways that stimulate enzyme activity for glucose release.
c. Insulin:
- Produced by: Beta cells of the pancreas (islets of Langerhans).
- Function: Lowers blood glucose levels by facilitating cellular glucose uptake, promoting glycogenesis (formation of glycogen), and inhibiting gluconeogenesis.
- Mechanism: Insulin binds to insulin receptors on cells, initiating a signaling cascade that results in the insertion of glucose transporters (e.g., GLUT4) into the cell membrane, enhancing glucose uptake.
2. Comparison of the Nervous System and the Endocrine System
| Feature | Nervous System | Endocrine System |
|---|---|---|
| Mode of Communication | Electrical impulses and neurotransmitters | Hormones released into the bloodstream |
| Speed of Response | Fast (milliseconds to seconds) | Slow (seconds to hours) |
| Duration of Effect | Short-lived | Long-lasting |
| Target Specificity | Highly specific (direct synaptic connections) | Less specific (hormones can affect multiple tissues) |
| Control | Primarily under voluntary control (somatic) and some involuntary (autonomic) | Primarily involuntary |
| Regulatory Mechanism | Negative feedback loops with rapid adjustments | Negative and positive feedback loops with gradual adjustments |
| Components | Neurons, synapses, central and peripheral structures | Glands (e.g., pituitary, thyroid, adrenal) |
Key Differences:
- Transmission: Nervous system uses electrical and chemical signals (neurotransmitters), whereas the endocrine system uses hormonal signals.
- Response Time: Nervous responses are immediate, suitable for quick actions (e.g., reflexes), while endocrine responses are slower, regulating long-term processes (e.g., growth, metabolism).
- Effect Specificity: Nervous signals target specific cells via synapses; hormones can have widespread effects on various tissues and organs.
3. Structure and Function of Sensory Neurones, Motor Neurones, and Intermediate Neurones
Neuronal Types:
a. Sensory Neurones:
- Structure:
- Dendrites: Receive stimuli from sensory receptors.
- Cell Body (Soma): Contains nucleus and organelles.
- Axon: Transmits impulses towards the central nervous system (CNS).
- Myelin Sheath: Often present, facilitating faster impulse transmission.
- Function: Detect external or internal stimuli (e.g., touch, temperature, pain) and convert them into electrical impulses.
b. Motor Neurones:
- Structure:
- Dendrites: Receive impulses from the CNS.
- Cell Body (Soma): Contains nucleus and organelles.
- Axon: Transmits impulses away from the CNS to effector organs (muscles or glands).
- Myelin Sheath: Often present for rapid impulse transmission.
- Function: Convey signals from the CNS to muscles or glands, triggering responses such as muscle contraction or hormone secretion.
c. Intermediate (Inter) Neurones:
- Structure:
- Dendrites and Axons: Connect sensory and motor neurones within the CNS.
- Cell Body (Soma): Located within the CNS (brain and spinal cord).
- Function: Facilitate communication between sensory and motor neurones, integrating and processing information.
Neuronal Connections:
- Sensory Neurone → Intermediate Neurone → Motor Neurone: This pathway allows the transmission of sensory information to the CNS and the subsequent generation of appropriate motor responses.
4. Role of Sensory Receptor Cells in Detecting Stimuli and Stimulating Transmission of Impulses
Sensory Receptor Cells:
- Specialized cells located in sensory organs (e.g., eyes, ears, skin, taste buds) that detect specific types of stimuli.
- Types of Stimuli:
- Mechanical: Pressure, touch, sound.
- Chemical: Taste, smell.
- Thermal: Temperature changes.
- Photonic: Light.
Detection and Transduction:
- Stimulus Detection: Sensory receptors respond to specific external or internal stimuli.
- Transduction: Conversion of the stimulus into an electrical signal (nerve impulse) through changes in receptor cell membrane potential.
- Transmission: The electrical signal is transmitted to sensory neurones, initiating an action potential that travels towards the CNS.
Example – Photoreceptors in the Eye:
- Rods and Cones: Detect light intensity and color, respectively.
- Mechanism: Light alters the conformation of photopigments, leading to hyperpolarization of the receptor cell and modulation of neurotransmitter release, triggering an action potential in the connected sensory neurone.
5. Sequence of Events Leading to an Action Potential in a Sensory Neurone: Chemoreceptor in a Human Taste Bud
Example: Chemoreceptor in Taste Bud
Sequence of Events:
- Stimulus Detection:
- Chemical Stimuli: Molecules from food dissolve in saliva and interact with chemoreceptors on taste bud cells.
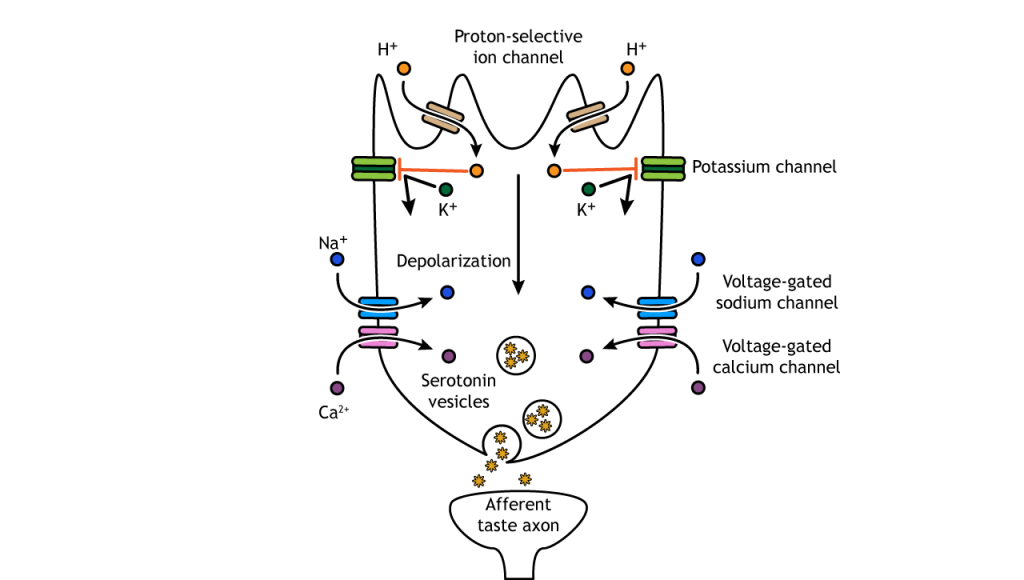
- Receptor Activation:
- Binding: Taste molecules bind to specific receptors on the chemoreceptor cell membrane.
- Conformational Change: Binding induces a change in receptor shape, leading to the opening of ion channels.
- Depolarization:
- Ion Movement: Influx of sodium ions (Na⁺) into the receptor cell causes depolarization.
- Threshold Reached: If depolarization reaches a certain threshold, voltage-gated sodium channels on the sensory neurone open.
- Action Potential Generation:
- Rapid Depolarization: Massive influx of Na⁺ due to opening of voltage-gated channels.
- Propagation: The action potential propagates along the sensory neurone towards the CNS.
- Transmission to CNS:
- Synapse: The action potential reaches the synapse, releasing neurotransmitters that cross to the next neuron (intermediate neurone).
Illustrative Diagram:
Taste bud with chemoreceptor cells interacting with taste molecules, leading to depolarization and action potential in sensory neurones.
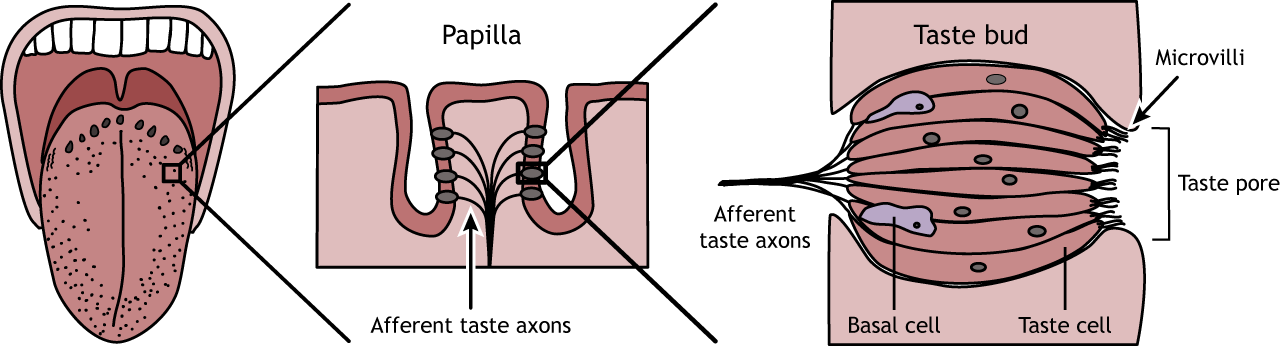
6. Changes to the Membrane Potential of Neurones
a. Maintaining the Resting Potential:
- Resting Potential: Typically around -70 mV inside the neuron relative to the outside.
- Ion Distribution:
- High K⁺ inside, high Na⁺ outside.
- Mechanism:
- Sodium-Potassium Pump (Na⁺/K⁺ ATPase): Actively transports 3 Na⁺ out and 2 K⁺ into the neuron using ATP, maintaining concentration gradients.
- Leak Channels: Allow K⁺ to diffuse out and Na⁺ to diffuse in passively, but the pump compensates to sustain the resting potential.
b. Events During an Action Potential:
- Depolarization:
- Stimulus: Threshold reached (-55 mV).
- Voltage-Gated Na⁺ Channels Open: Rapid influx of Na⁺, membrane potential becomes positive (+30 mV).
- Repolarization:
- Na⁺ Channels Inactivate: Stop Na⁺ influx.
- Voltage-Gated K⁺ Channels Open: K⁺ efflux restores negative membrane potential.
- Hyperpolarization:
- K⁺ Channels Remain Open Slightly Longer: Membrane potential becomes slightly more negative than resting (-80 mV).
c. Restoring the Resting Potential During the Refractory Period:
- Refractory Period: Phase following an action potential when the neuron cannot fire another action potential immediately.
- Mechanism:
- Sodium-Potassium Pump: Restores original ion distribution by pumping Na⁺ out and K⁺ in.
- Leak Channels Close: Stabilize the membrane potential back to resting state.
Summary:
- Resting Potential Maintenance: Na⁺/K⁺ pump and selective permeability.
- Action Potential Phases: Depolarization, repolarization, hyperpolarization.
- Refractory Period: Ensures unidirectional and discrete impulses.
7. Rapid Transmission of an Impulse in a Myelinated Neurone: Saltatory Conduction
Myelinated Neurones:
- Structure:
- Axon Covered with Myelin Sheath: Formed by Schwann cells in the PNS and oligodendrocytes in the CNS.
- Nodes of Ranvier: Gaps (~1 mm) between adjacent Schwann cells where voltage-gated channels are concentrated.
Saltatory Conduction:
- Mechanism:
- Impulse Jumps: Action potential “jumps” from one Node of Ranvier to the next, bypassing the myelinated sections.
- Advantages:
- Speed: Significantly faster transmission compared to unmyelinated fibres.
- Energy Efficiency: Fewer ion exchanges reduce the metabolic cost of maintaining ion gradients.
Process:
- Action Potential Initiation: At the axon hillock.
- Jumping Between Nodes: Rapid depolarization at Nodes of Ranvier propagates the impulse along the axon.
- Continuous Cycle: The process repeats at each node, ensuring swift and efficient impulse travel.
Illustrative Diagram:
Myelinated axon with Nodes of Ranvier, where the action potential jumps between nodes, demonstrating saltatory conduction.
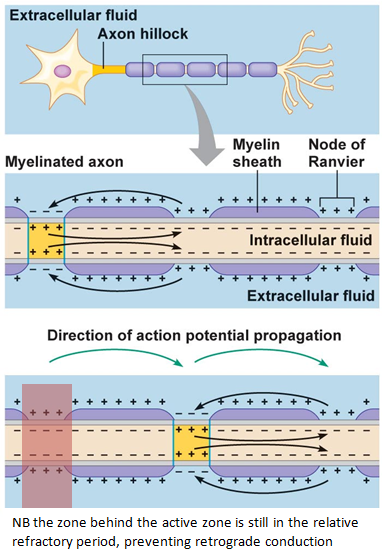
8. Importance of the Refractory Period in Determining the Frequency of Impulses
Refractory Period:
- Definition: The time after an action potential during which a neuron cannot fire another action potential or requires a stronger stimulus to do so.
- Types:
- Absolute Refractory Period: No possibility of initiating another action potential; voltage-gated Na⁺ channels are inactivated.
- Relative Refractory Period: A stronger-than-usual stimulus can initiate an action potential; some K⁺ channels are still open.
Role in Impulse Frequency:
- Limits Firing Rate: The refractory period sets a maximum frequency at which neurons can fire action potentials.
- Prevents Overlapping: Ensures that each action potential is distinct and unidirectional.
- Determines Temporal Resolution: Influences how rapidly neurons can respond to successive stimuli, affecting processes like muscle contraction speed and sensory perception.
Example:
- Rapid Impulse Generation: Short refractory periods allow neurons to fire at high frequencies, essential for activities requiring quick responses (e.g., sprinting).
- Slower Activities: Longer refractory periods prevent fatigue and overexcitation, maintaining neuronal health.
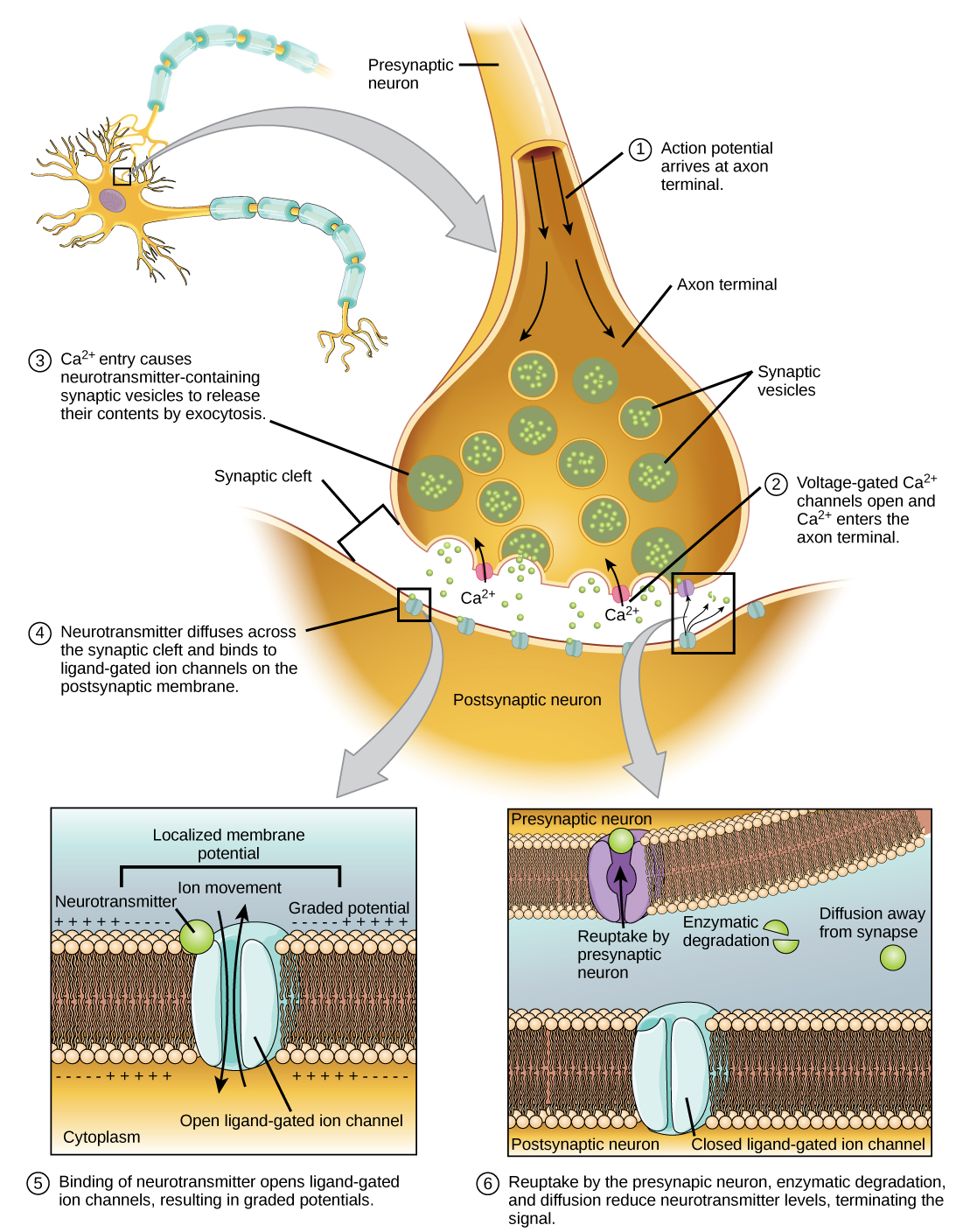
9. Structure and Function of a Cholinergic Synapse: Role of Calcium Ions
Cholinergic Synapse:
- Definition: A synapse where the neurotransmitter acetylcholine (ACh) is used.
- Components:
- Presynaptic Terminal: Contains synaptic vesicles filled with ACh.
- Synaptic Cleft: Gap between neurons (~20-40 nm wide).
- Postsynaptic Membrane: Contains ACh receptors (e.g., nicotinic receptors) linked to ion channels.
Function:
- Action Potential Arrival: Reaches the presynaptic terminal.
- Calcium Influx:
- Voltage-Gated Ca²⁺ Channels Open: Ca²⁺ enters the presynaptic terminal.
- Vesicle Fusion:
- Calcium Triggers Exocytosis: Synaptic vesicles fuse with the presynaptic membrane, releasing ACh into the synaptic cleft.
- Neurotransmitter Binding:
- ACh Binds to Receptors: Opens ion channels on the postsynaptic membrane, leading to depolarization (excitatory postsynaptic potential).
- Termination of Signal:
- ACh Breakdown: Acetylcholinesterase enzyme degrades ACh, terminating the signal and allowing the neuron to reset.
Role of Calcium Ions:
- Essential for Vesicle Fusion: Ca²⁺ binds to proteins involved in vesicle docking and fusion, facilitating neurotransmitter release.
- Signal Transduction: Acts as a secondary messenger initiating the cascade leading to exocytosis.
Illustrative Diagram:
A synapse with presynaptic terminal releasing ACh upon Ca²⁺ influx, ACh crossing the synaptic cleft, binding to postsynaptic receptors, and terminating via acetylcholinesterase.
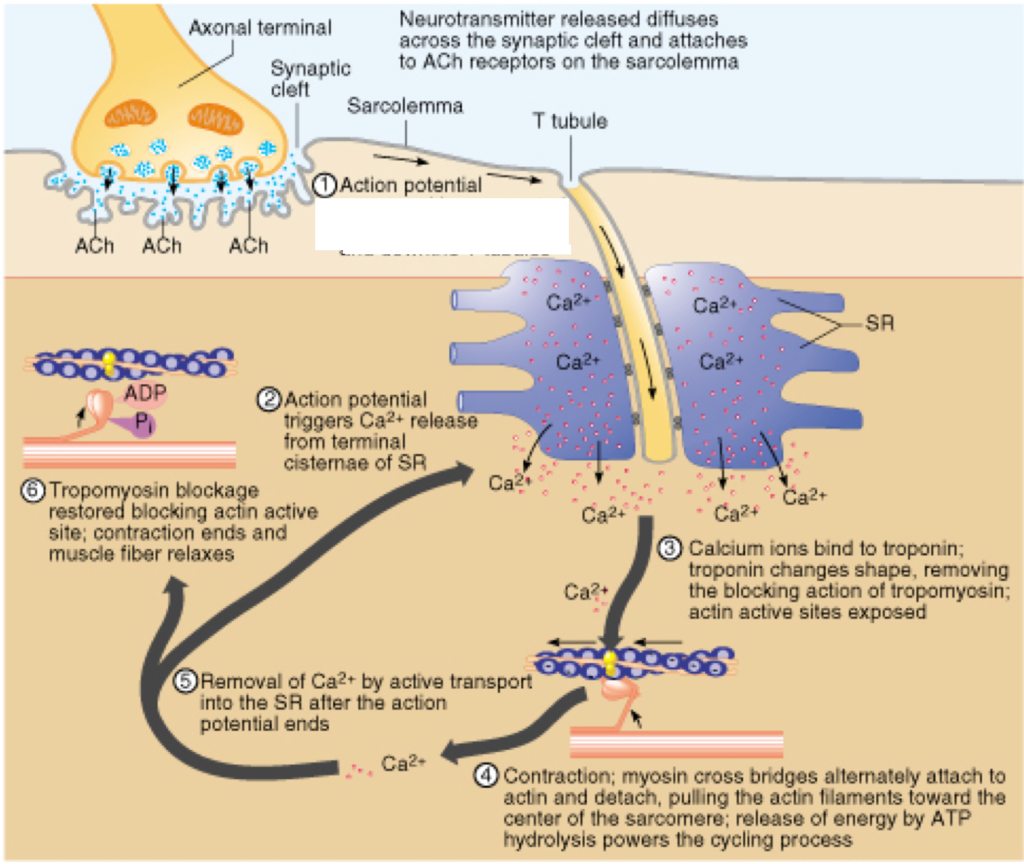
10. Roles of Neuromuscular Junctions, T-Tubule System, and Sarcoplasmic Reticulum in Stimulating Contraction in Striated Muscle
a. Neuromuscular Junction (NMJ):
- Definition: Synapse between a motor neurone and a skeletal muscle fibre.
- Function:
- Transmission of Impulse: Motor neurone releases ACh into the NMJ.
- Muscle Activation: ACh binds to receptors on the muscle fibre, generating an action potential that initiates muscle contraction.
b. T-Tubule System:
- Structure: Transverse (T) tubules are invaginations of the muscle fibre’s plasma membrane (sarcolemma) that penetrate into the cell’s interior.
- Function:
- Impulse Transmission: Conducts action potentials from the sarcolemma deep into the muscle fibre.
- Synchronization: Ensures that the action potential reaches all regions of the muscle fibre simultaneously, promoting coordinated contraction.
c. Sarcoplasmic Reticulum (SR):
- Structure: A specialized form of endoplasmic reticulum surrounding each myofibril, forming a network of tubules and cisternae.
- Function:
- Calcium Storage: Stores Ca²⁺ ions within its lumen.
- Calcium Release: Action potentials traveling through T-tubules trigger the SR to release Ca²⁺ into the sarcoplasm.
- Contraction Initiation: Increased Ca²⁺ concentration enables the sliding filament mechanism by binding to troponin.
Integrated Process of Muscle Contraction:
- NMJ Activation: Motor neurone releases ACh, triggering an action potential in the muscle fibre.
- Impulse Propagation: Action potential travels along the sarcolemma and down T-tubules.
- Calcium Release: T-tubule action potential stimulates SR to release Ca²⁺.
- Sliding Filament Mechanism: Ca²⁺ binds to troponin, leading to muscle contraction.
Illustrative Diagram:
A neuromuscular junction with motor neurone releasing ACh, action potential traveling through T-tubules, and SR releasing Ca²⁺ to initiate contraction.
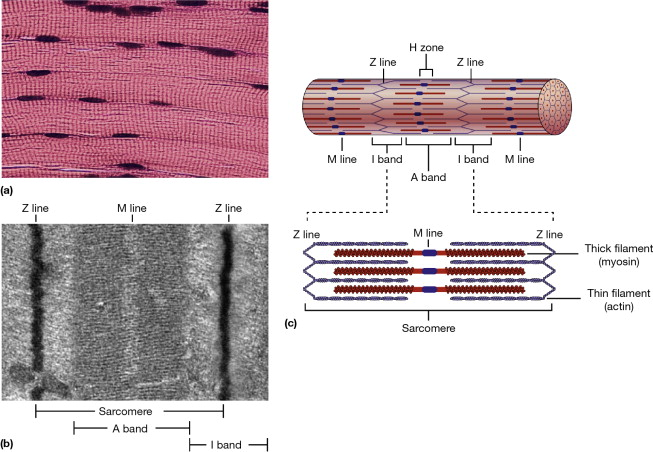
11. Ultrastructure of Striated Muscle: Sarcomere Structure
Striated Muscle Overview:
- Types: Skeletal and cardiac muscles.
- Appearance: Striations due to the organized arrangement of myofilaments.
Sarcomere Structure:
- Definition: The basic functional unit of a muscle fibre, bounded by Z-lines (Z-discs).
- Components:
- Thin Filaments: Primarily actin, associated with troponin and tropomyosin.
- Thick Filaments: Primarily myosin.
- Z-Lines: Anchor the thin filaments and define the boundaries of each sarcomere.
- A Band: Dark region containing overlapping thick and thin filaments.
- I Band: Light region containing only thin filaments.
- H Zone: Central part of the A band with only thick filaments.
- M Line: Center of the H zone, where myosin filaments are anchored.
Ultrastructure:
- Electron Micrographs: Reveal the detailed arrangement of myofilaments within the sarcomere.
- Organization:
- Myofibrils: Composed of repeating sarcomeres arranged end-to-end.
- Intercalated Discs (in cardiac muscle): Specialized connections facilitating synchronized contraction.
Functional Significance:
- Contractile Units: Sarcomeres shorten during contraction, pulling Z-lines closer and shortening the entire muscle fibre.
- Striated Appearance: Results from the precise alignment of thick and thin filaments within sarcomeres.
Illustrative Diagram Description:
A sarcomere with Z-lines at each end, thick myosin filaments and thin actin filaments interdigitating within the A band, a lighter I band, and the H zone in the center. Myofilaments are organized in a regular, repeating pattern giving striated muscle its characteristic appearance.
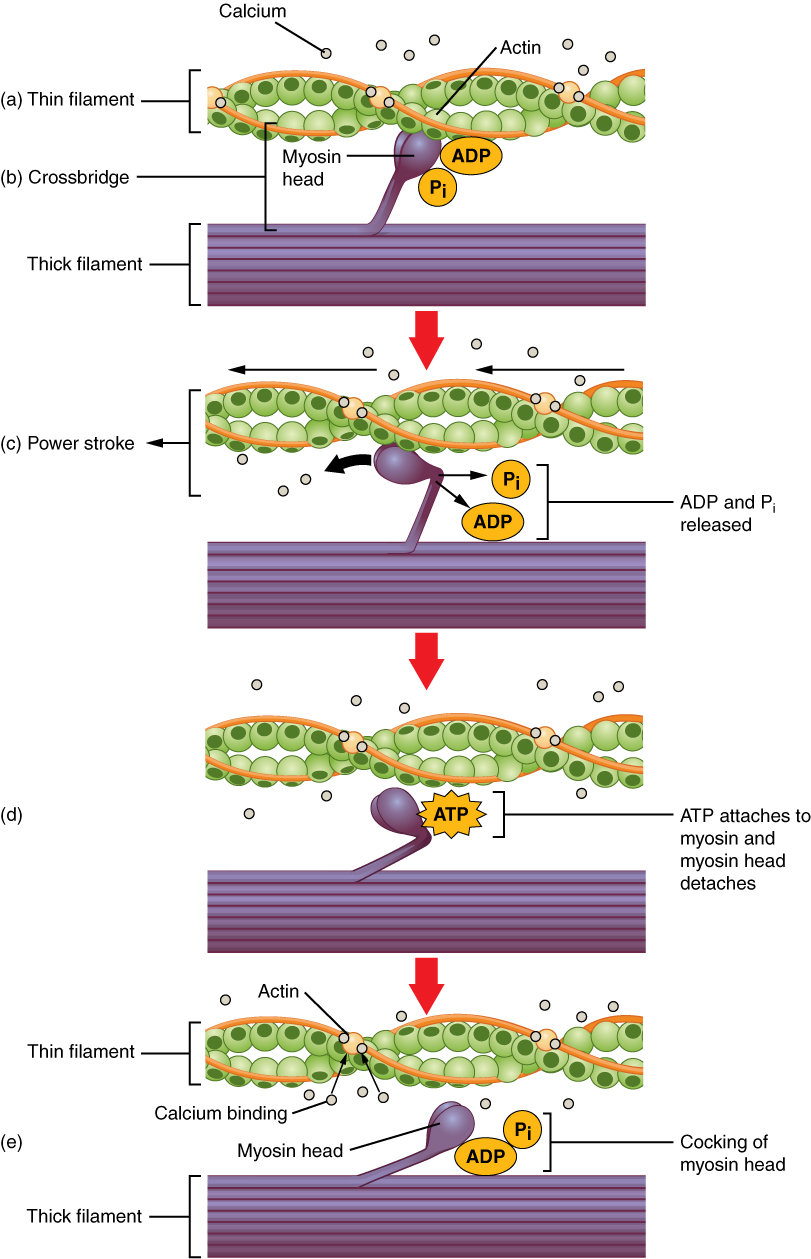
12. Sliding Filament Model of Muscular Contraction
Sliding Filament Theory:
- Concept: Muscle contraction occurs through the sliding of thin (actin) and thick (myosin) filaments past each other without changing their lengths.
- Key Components:
- Actin (Thin Filaments): Contain binding sites for myosin heads.
- Myosin (Thick Filaments): Possess heads that form cross-bridges with actin.
- Troponin and Tropomyosin: Regulatory proteins on actin that control myosin binding.
Sequence of Events:
- Calcium Release:
- SR Releases Ca²⁺: Action potential triggers SR to release Ca²⁺ into the sarcoplasm.
- Binding of Ca²⁺ to Troponin:
- Conformational Change: Ca²⁺ binds to troponin, causing tropomyosin to shift and expose myosin-binding sites on actin.
- Cross-Bridge Formation:
- Myosin Heads Attach: Myosin heads bind to exposed sites on actin, forming cross-bridges.
- Power Stroke:
- ATP Hydrolysis: Myosin heads hydrolyze ATP to ADP and Pi, providing energy.
- Movement: Myosin heads pivot, pulling actin filaments towards the center of the sarcomere, shortening the muscle.
- Cross-Bridge Detachment:
- ATP Binding: A new ATP molecule binds to myosin, causing it to detach from actin.
- Resetting Myosin Heads:
- ATP Hydrolysis: Myosin hydrolyzes ATP to reposition for another cycle.
Role of ATP:
- Energy Source: Provides the necessary energy for myosin head movement and detachment from actin.
- Recycling: Ensures continuous cycling of cross-bridge formation and detachment during sustained muscle contraction.
Role of Regulatory Proteins:
- Troponin: Binds Ca²⁺, initiating the exposure of binding sites.
- Tropomyosin: Blocks myosin-binding sites in the absence of Ca²⁺, preventing unintended contraction.
Summary:
- Contraction Mechanism: Repeated cycles of cross-bridge attachment, power stroke, and detachment result in the sliding of filaments and muscle shortening.
- Regulation: Controlled by Ca²⁺ levels and ATP availability, ensuring precise and efficient muscle contractions.
Illustrative Diagram Description:
Overlapping thin and thick filaments within a sarcomere. Myosin heads form cross-bridges with actin, pivoting to pull actin towards the M line during the power stroke, resulting in sarcomere shortening.
13. Rapid Response of the Venus Flytrap to Stimulation of Hairs on the Lobes of Modified Leaves
a. Structure of the Venus Flytrap:
- Modified Leaves: The Venus flytrap (Dionaea muscipula) has specialized leaves divided into two lobes hinged together. Each lobe has hair-like structures called trigger hairs on the inner surfaces.
- Snap Trap Mechanism: The edges of the lobes have interlocking teeth that form a trap when closed.
b. Mechanism of Trap Closure:
- Stimulation of Trigger Hairs:
- When an insect or other small prey touches the trigger hairs located on the inner surfaces of the lobes, it sends an electrical signal.
- Threshold Stimulation: At least two stimulations within a short time (e.g., 20 seconds) are required to trigger the closure to avoid false alarms (e.g., debris).
- Electrical Signal Transmission:
- The stimulation induces a rapid change in turgor pressure within the cells of the lobes.
- Action Potential: Similar to nerve impulses in animals, an action potential travels across the lobe tissues.
- Turgor Pressure Changes:
- Ionic Flux: Movement of ions (e.g., potassium ions) into and out of cells alters the osmotic balance.
- Water Movement: Water follows ions, leading to a loss of turgor pressure in specific cells.
- Bend and Closure:
- Elastic Structures: The lobe tissues have elastic properties that cause the lobes to bend inward rapidly.
- Trap Closure: The speed of closure can be as fast as 100 milliseconds, effectively trapping the prey inside.
- Post-Closure Sealing:
- Locking Mechanism: After closure, the trap locks to prevent escape.
- Enzyme Secretion: Digestive enzymes are secreted to break down the prey over several days.
c. Significance:
- Carnivorous Adaptation: The rapid closure allows the plant to capture insects for essential nutrients, especially nitrogen, in nutrient-poor environments.
14. Role of Auxin in Elongation Growth by Stimulating Proton Pumping to Acidify Cell Walls
a. Auxin Overview:
- Definition: Auxins are a class of plant hormones (e.g., Indole-3-acetic acid, IAA) that regulate various aspects of growth and development.
- Production Sites: Primarily produced in young leaves, apical buds, and root tips.
b. Acid Growth Theory (Peter and Franklin, 1973):
- Mechanism:
- Auxin Transport: Auxin is transported from the shoot apex downward through the stem in a concentration gradient.
- Proton Pump Activation: Auxin binds to receptor proteins on the plasma membrane, activating H⁺-ATPase proton pumps.
- Cell Wall Acidification: Activated proton pumps expel H⁺ ions into the cell wall (apoplast), lowering the pH (acidifying the cell wall).
- Effects of Acidification:
- Hydrogen Bond Disruption: Lower pH protonates expansins, proteins that loosen hydrogen bonds between cellulose fibers in the cell wall.
- Cell Wall Loosening: The loosened cell wall allows for turgor-driven expansion, enabling the cell to elongate.
- Elongation Growth: Sustained cell elongation results in overall plant growth, particularly in stems and shoots.
c. Summary of Steps:
- Auxin concentration increases on the shaded side of a plant stem.
- Auxin activates proton pumps, leading to acidification of the cell wall.
- Acidified cell walls activate expansins, loosening the wall structure.
- Turgor pressure causes the cell to elongate, resulting in bending towards light (phototropism).
d. Significance:
- Plant Morphogenesis: Auxin-mediated cell elongation is crucial for plant responses to environmental stimuli, such as light and gravity.
- Adaptive Growth: Allows plants to optimize light capture and stabilize their structure.
15. Role of Gibberellin in the Germination of Barley
a. Gibberellin Overview:
- Definition: Gibberellins (GAs) are a group of plant hormones that promote various growth processes, including seed germination, stem elongation, and flowering.
- Biosynthesis: Produced in the embryo of seeds and in young leaves.
b. Germination Process in Barley:
- Seed Dormancy and Activation:
- Dormant State: Seeds remain dormant until conditions (water, temperature) are favorable for germination.
- Activation: Water uptake (imbibition) reactivates metabolic processes.
- Gibberellin Production:
- Synthesis Trigger: Imbibition and hormonal signaling stimulate the synthesis of gibberellin in the embryo.
- Transport: Gibberellins are transported to the aleurone layer of the seed.
- Action on Aleurone Layer:
- Enzyme Induction: Gibberellins induce the synthesis and secretion of hydrolytic enzymes (e.g., α-amylase).
- Nutrient Mobilization: Enzymes break down starches into sugars, providing energy and building blocks for the growing embryo.
- Radicle and Plumule Growth:
- Turgor Pressure Increase: Gibberellins promote cell elongation in the radicle (embryonic root) and plumule (embryonic shoot).
- Emergence: The radicle emerges first, anchoring the seed, followed by the plumule, which grows towards the light.
- Overcoming Dormancy:
- Balance with ABA: Abscisic acid (ABA) inhibits germination; gibberellin and ABA levels are balanced to regulate timing.
- Environmental Cues: Gibberellin synthesis is influenced by light, temperature, and water availability.
c. Experimental Evidence:
- GA-Deficient Mutants: Barley mutants deficient in gibberellin fail to germinate properly, confirming the hormone’s essential role.
- Exogenous Application: Applying gibberellin to dormant seeds can break dormancy and induce germination.
d. Summary of Gibberellin Function:
- Stimulates enzyme production in the aleurone layer.
- Mobilizes stored nutrients for embryo growth.
- Promotes cell elongation in the emerging root and shoot.
- Facilitates the transition from seed dormancy to active growth.
e. Significance:
- Agricultural Importance: Understanding gibberellin’s role aids in improving crop germination rates and vigor.
- Biotechnological Applications: Manipulation of gibberellin levels can enhance seedling growth and stress responses.
Key Terms and Concepts
- Action Potential: Rapid electrical signal that propagates along the plant tissue in response to stimuli.
- Turgor Pressure: Pressure exerted by the fluid inside the cell against the cell wall, essential for maintaining plant rigidity and driving cell expansion.
- Expansins: Proteins that loosen the plant cell wall, allowing for cell expansion and growth.
- Aleurone Layer: Outermost layer of the endosperm in cereal grains, involved in nutrient mobilization during germination.
- Phototropism: Growth of plants in response to light direction, often mediated by auxin distribution.
- Hydrolytic Enzymes: Enzymes that catalyze the breakdown of complex molecules into simpler ones (e.g., α-amylase breaks down starch into sugars).
Practise Questions
Test 1
1. What characterizes endocrine glands?
2. Which type of neurone transmits impulses from receptors to the central nervous system (CNS)?
3. What is the primary function of myofibrils in striated muscle?
4. Which plant hormone is primarily responsible for promoting cell elongation?
5. During muscle contraction, what role does ATP play?
6. What is the primary difference between saltatory and continuous transmission in neurones?
7. Which hormone is involved in plant responses to environmental stress, such as stomatal closure during drought?
8. What occurs during the depolarization phase of an action potential in neurones?
9. In the neuromuscular junction, which neurotransmitter is released to initiate muscle contraction?
10. What is the role of expansins in plant cell elongation?
Correct Answers: 0%
Test 2
1. How do insulin and glucagon function as a negative feedback mechanism to regulate blood glucose levels?
2. What role does Antidiuretic Hormone (ADH) play during dehydration, and how does it achieve this?
3. In the context of action potential propagation, explain the difference between saltatory and continuous transmission.
4. Describe the role of troponin in the regulation of muscle contraction.
5. Explain how gibberellin facilitates seed germination in barley.
6. Compare the speed and duration of responses mediated by the endocrine system and the nervous system.
7. What is the function of intermediate (relay) neurones in the central nervous system?
8. How does the Venus Fly Trap utilize electrical communication in response to mechanical stimulation?
9. Describe the process that leads to muscle relaxation after contraction.
10. What is the primary function of expansins in plant cell elongation?
Correct Answers: 0%
Test 3
1. How do insulin and glucagon function as a negative feedback mechanism to regulate blood glucose levels?
2. What role does Antidiuretic Hormone (ADH) play during dehydration, and how does it achieve this?
3. In the context of action potential propagation, explain the difference between saltatory and continuous transmission.
4. Describe the role of troponin in the regulation of muscle contraction.
5. Explain how gibberellin facilitates seed germination in barley.
6. Compare the speed and duration of responses mediated by the endocrine system and the nervous system.
7. What is the function of intermediate (relay) neurones in the central nervous system?
8. How does the Venus Fly Trap utilize electrical communication in response to mechanical stimulation?
9. Describe the process that leads to muscle relaxation after contraction.
10. What is the primary function of expansins in plant cell elongation?
Correct Answers: 0%
Test 4
1. What is the primary function of the endocrine system in coordinating body activities?
2. How do sensory receptor cells in taste buds transduce chemical signals into neural signals?
3. What mechanism ensures that action potentials propagate in only one direction along a neurone?
4. In striated muscle fibers, what defines the boundaries of a sarcomere?
5. How does the refractory period contribute to the unidirectional propagation of action potentials?
6. What role do DELLA proteins play in plant growth regulation?
7. During an action potential, what initiates the repolarization phase?
8. How does abscisic acid (ABA) help plants respond to drought conditions?
9. What is the function of the sarcoplasmic reticulum in muscle cells?
10. In plant cell elongation, how do expansins facilitate this process?
Correct Answers: 0%
Test 5
1. What triggers the release of antidiuretic hormone (ADH) from the posterior pituitary gland?
2. Which statement correctly describes the role of myelin sheath in nerve impulse transmission?
3. In synaptic transmission, what is the role of calcium ions (Ca²⁺) when an action potential arrives at the presynaptic terminal?
4. During muscle contraction, what initiates the power stroke of the myosin head?
5. Which energy source is used first during initial stages of muscle contraction before aerobic respiration increases?
6. How does auxin (IAA) cause phototropism in plant shoots?
7. What is the primary role of DELLA proteins in gibberellin signaling pathways in plants?
8. In the Venus Fly Trap, why are multiple stimulations of trigger hairs required before the trap closes?
9. How does abscisic acid (ABA) cause stomatal closure during water stress?
10. What differentiates endocrine glands from exocrine glands?
Correct Answers: 0%