08.00 Chapter Summary
1. Rates of Reaction
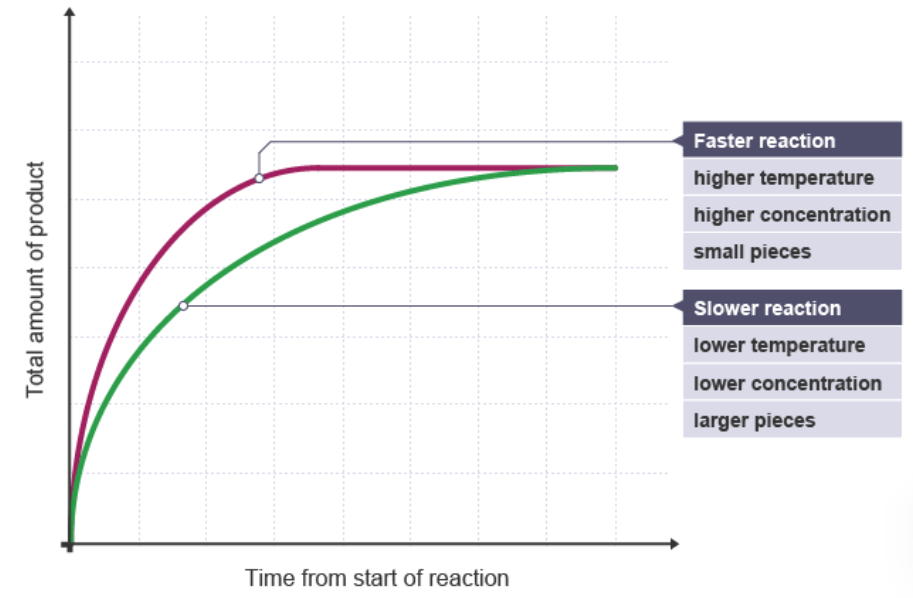
1.1 Factors Affecting Reaction Rates
Concentration of Reactants:
- Effect: Higher concentration increases the number of collisions, speeding up the reaction.
- Example: Doubling the concentration of hydrogen peroxide in its decomposition reaction doubles the rate.
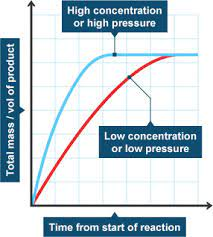
Pressure (for Gaseous Reactions):
- Effect: Increased pressure raises the concentration of gas molecules, leading to more frequent collisions.
- Example: Increasing the pressure in the Haber process (N₂ + 3H₂ ⇌ 2NH₃) shifts equilibrium to produce more ammonia.
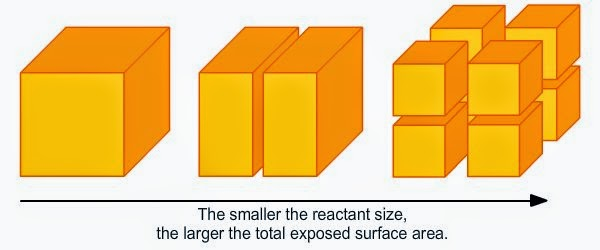
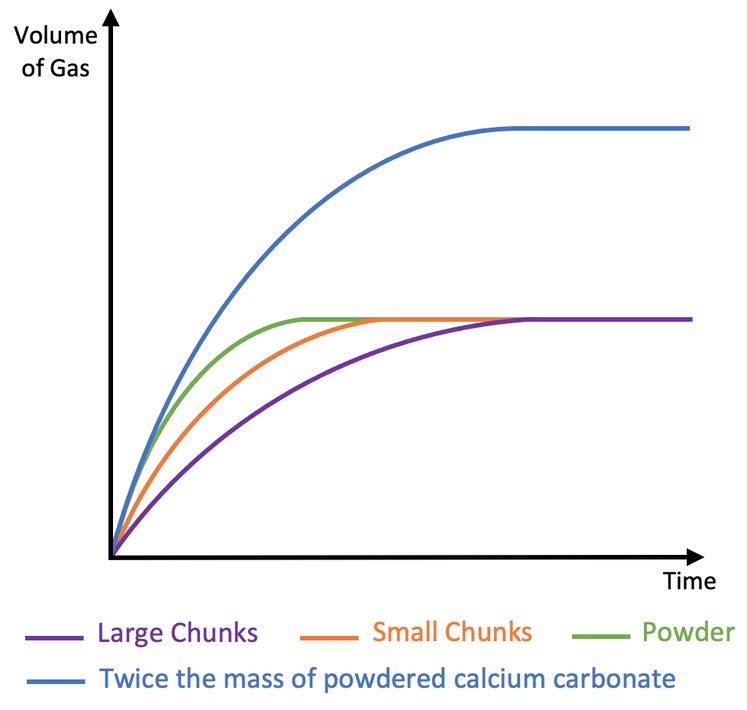
Surface Area of Solid Reactants:
- Effect: Greater surface area (e.g., powdered vs. lump solids) exposes more particles to reactants, increasing the reaction rate.
- Example: Powdered magnesium reacts faster with hydrochloric acid than magnesium ribbon.

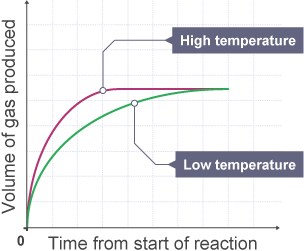
Temperature:
- Effect: Higher temperatures increase particle kinetic energy, resulting in more frequent and energetic collisions, thus speeding up the reaction.
- Example: Heating hydrochloric acid increases the rate at which magnesium reacts with it.
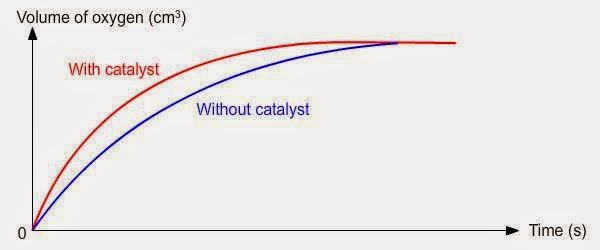
Presence of a Catalyst:
- Effect: Catalysts provide an alternative reaction pathway with lower activation energy, increasing the rate without being consumed.
- Example: Iron catalyst in the Haber process accelerates ammonia production.
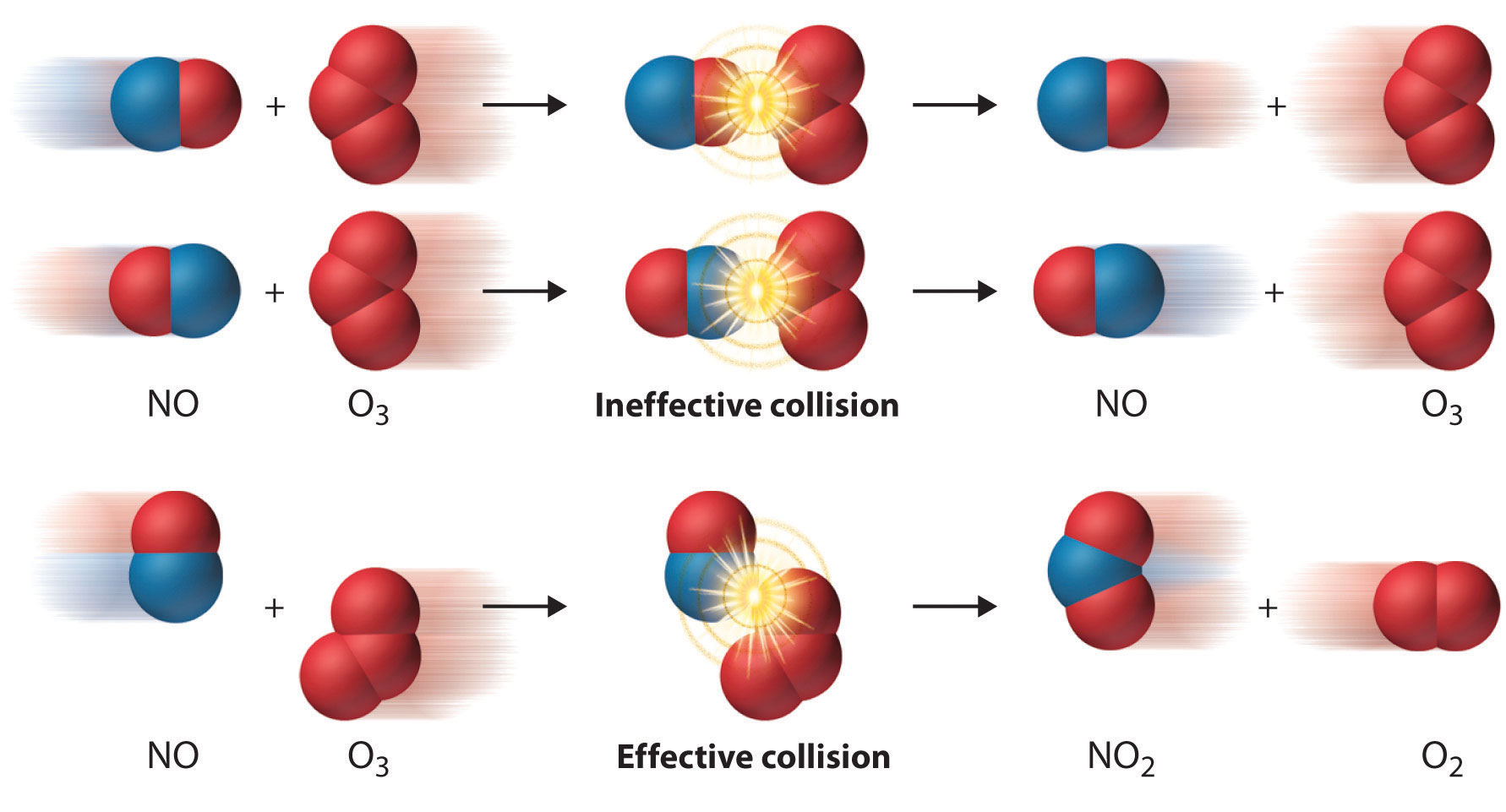
1.2 Collision Theory
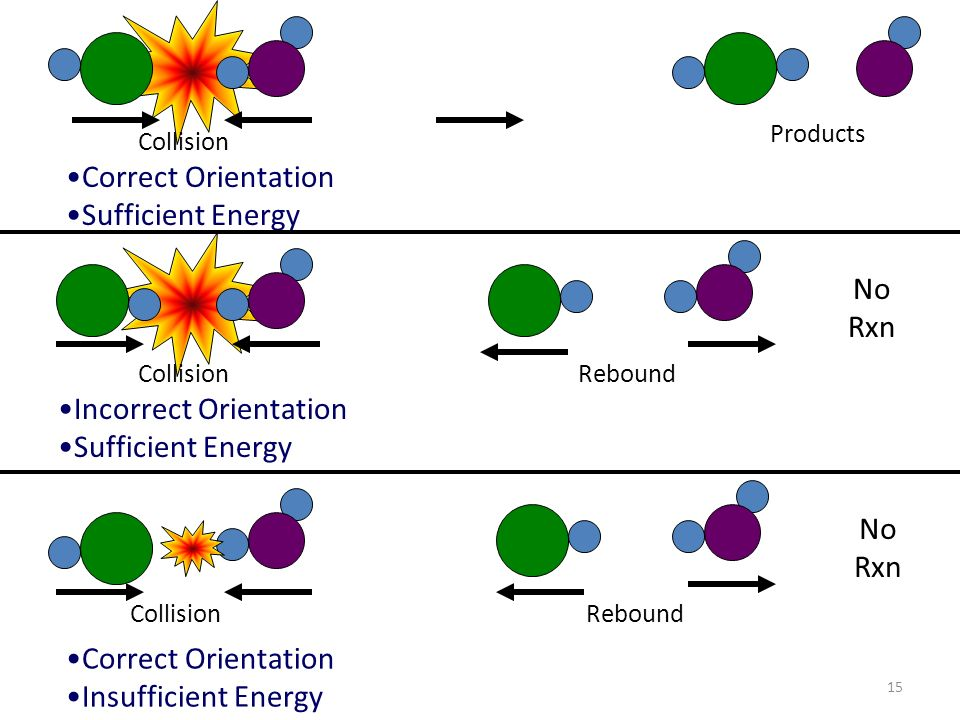
- Principles:
- Necessary Collisions: Reactant particles must collide to react.
- Sufficient Energy: Collisions must have enough energy (≥ activation energy) to break bonds.
- Proper Orientation: Particles must collide in the correct orientation for a reaction to occur.
- Types of Collisions:
- Successful Collisions: Lead to product formation.
- Unsuccessful Collisions: Particles bounce off without reacting.
1.3 Measuring Reaction Rates
Methods:
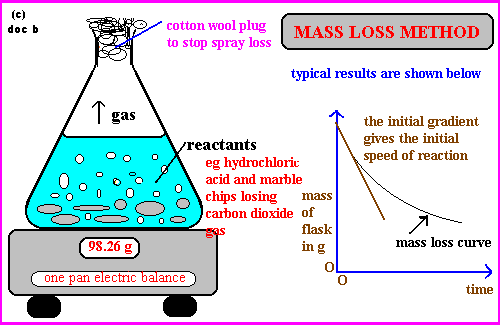
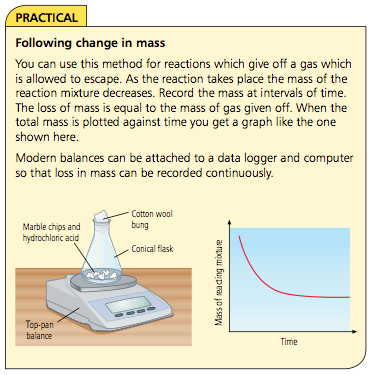
- Mass Loss: Monitoring mass loss if a gas is produced (e.g., magnesium reacting with hydrochloric acid).
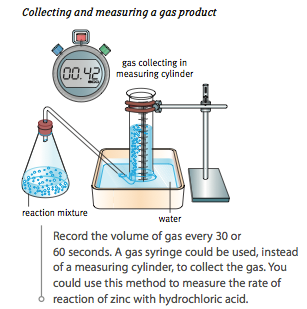
- Gas Volume: Collecting and measuring the volume of gas produced over time (e.g., decomposition of hydrogen peroxide).
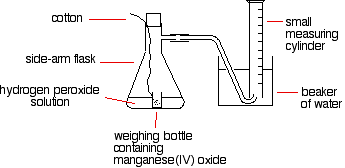
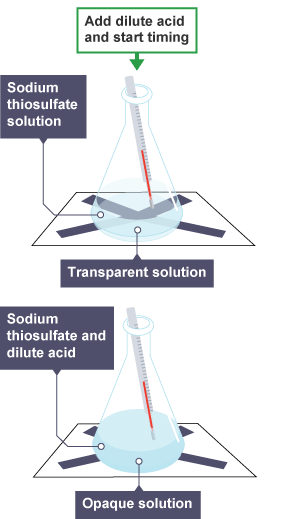
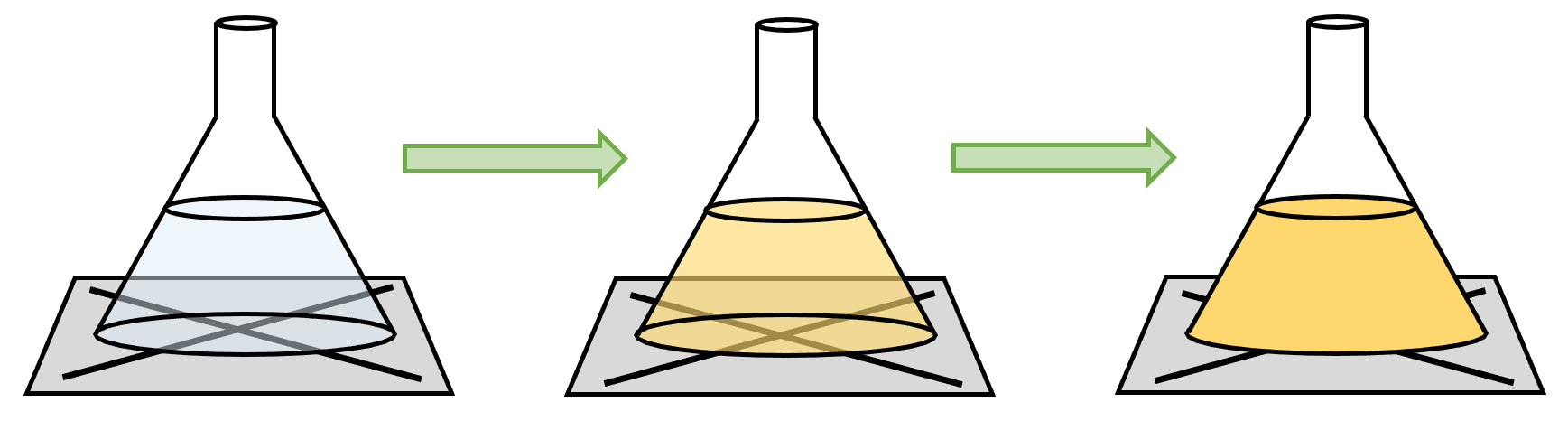
- Color Change: Timing how long a color change takes to occur (e.g., the disappearing cross experiment with sodium thiosulfate).
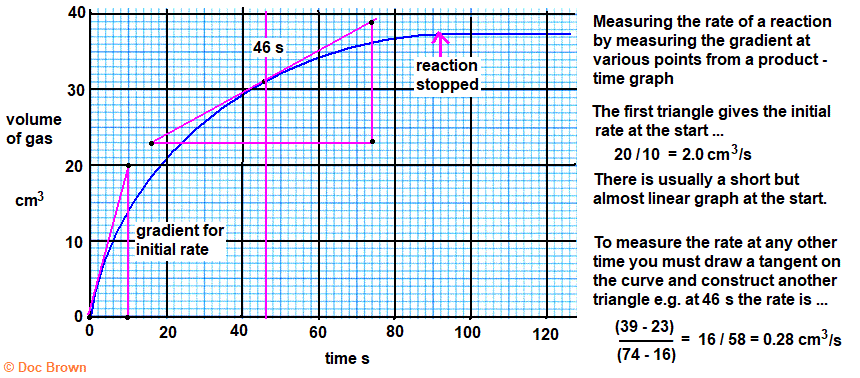
- Graphing Data:
- Rate vs. Time: Initial rate is steepest, decreasing as reactants are consumed.
- Tangents: Used to determine instantaneous rates at specific points.
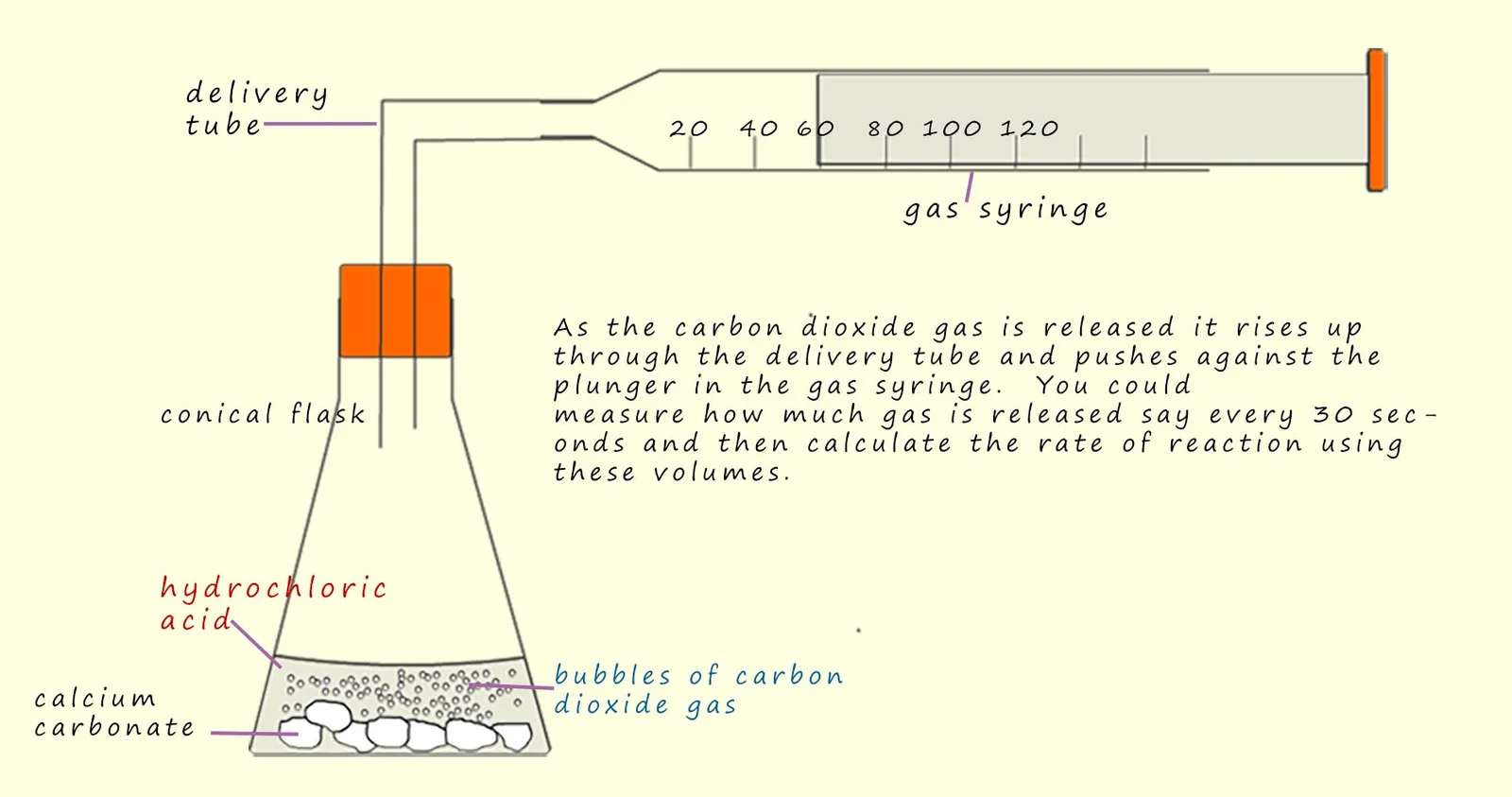
1.4 Evaluating Methods of Measuring Reaction Rates
- Gas Syringe:
- Advantages: Accurate, suitable for all gas-producing reactions.
- Disadvantages: Fragile, expensive, can stick, limited gas volume capacity.
- Inverted Measuring Cylinder:
- Advantages: Simple setup, uses common lab equipment.
- Disadvantages: Gas can be lost if not properly sealed, difficult to read upside-down.
- Mass Measurement:
- Advantages: Easy to set up, uses common equipment.
- Disadvantages: Not suitable for low-mass gases like hydrogen.
- Disappearing Cross Experiment:
- Advantages: Simple, no specialized equipment needed.
- Disadvantages: Subjective timing, potential for equipment contamination.
2. Physical vs. Chemical Changes
- Indicators of Chemical Changes:
- Formation of a precipitate.
- Evolution of gas (effervescence).
- Color change.
- Temperature change (exothermic/endothermic).
- Indicators of Physical Changes:
- Change in state or form without new substances.
2.2 Factors Affecting Reaction Rates
- Remember the Five Factors:
- Concentration
- Pressure (gases)
- Surface Area
- Temperature
- Catalysts
- Use the Collision Theory Framework: Focus on collision frequency and energy.
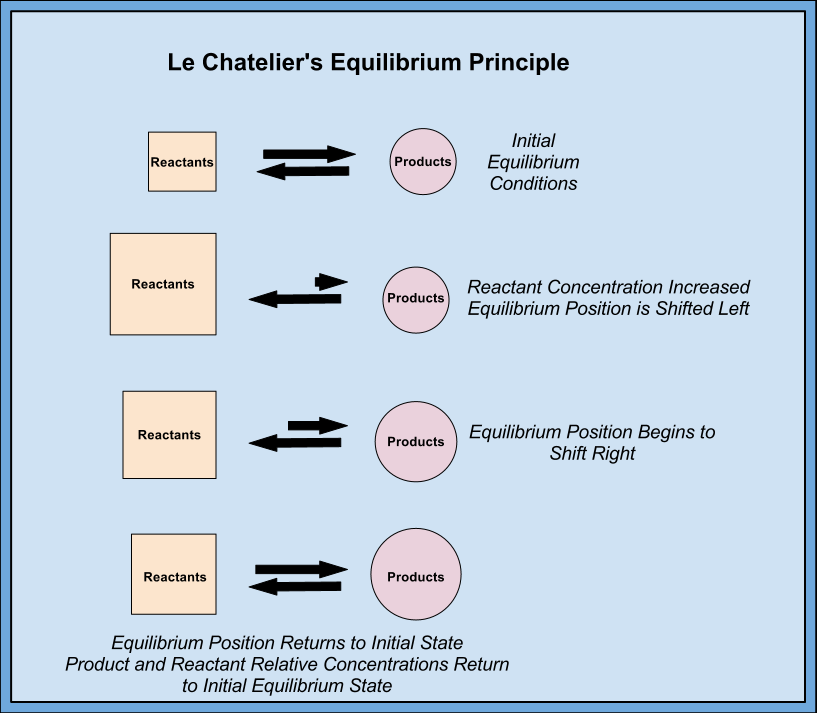
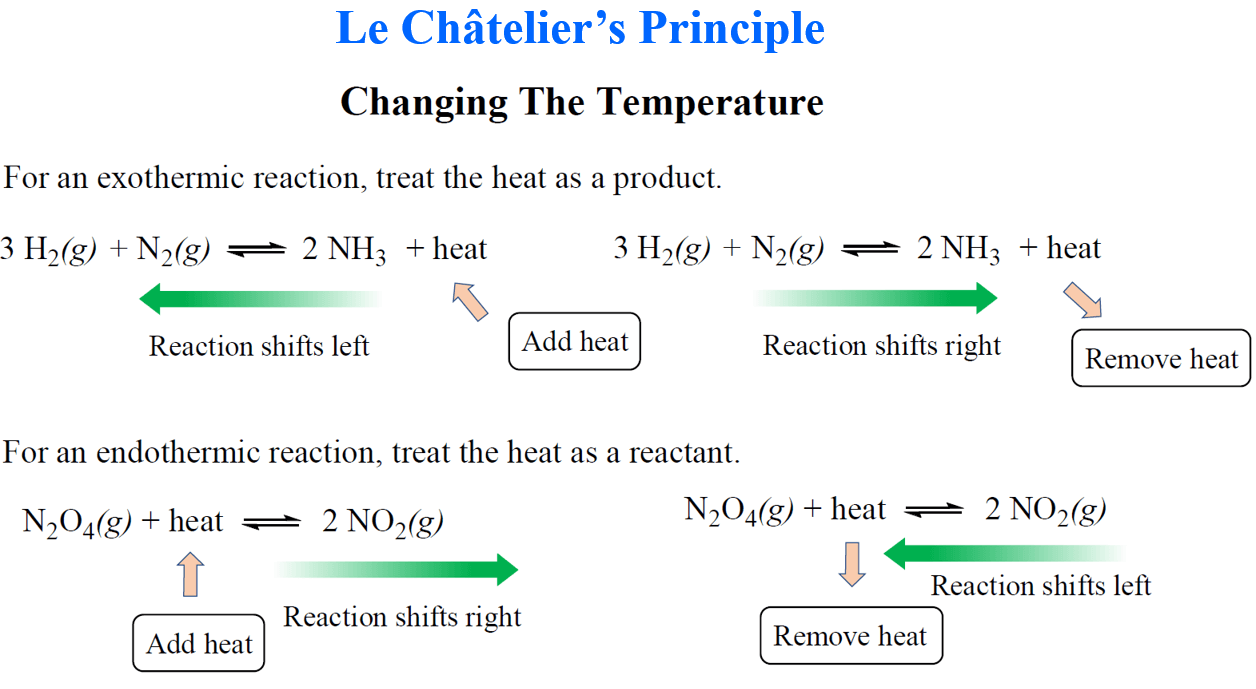
3. Equilibrium Shifts
- Use Le Chatelier’s Principle: Identify how changes affect the position of equilibrium.
- Predict Direction: Determine whether shifts favor reactants or products based on changes in concentration, pressure, or temperature.
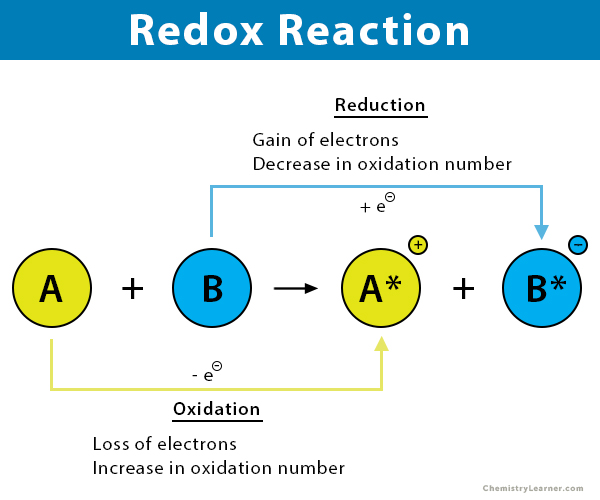
4. Redox Reactions
- Use Oxidation Numbers: Assign and track changes to identify oxidized and reduced species.
- Mnemonic:OIL-RIG
- Oxidation Is Loss (of electrons).
- Reduction Is Gain (of electrons).
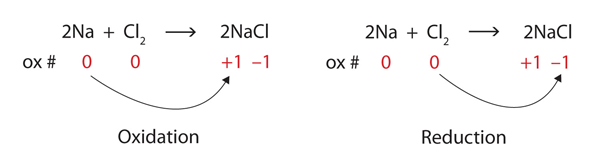
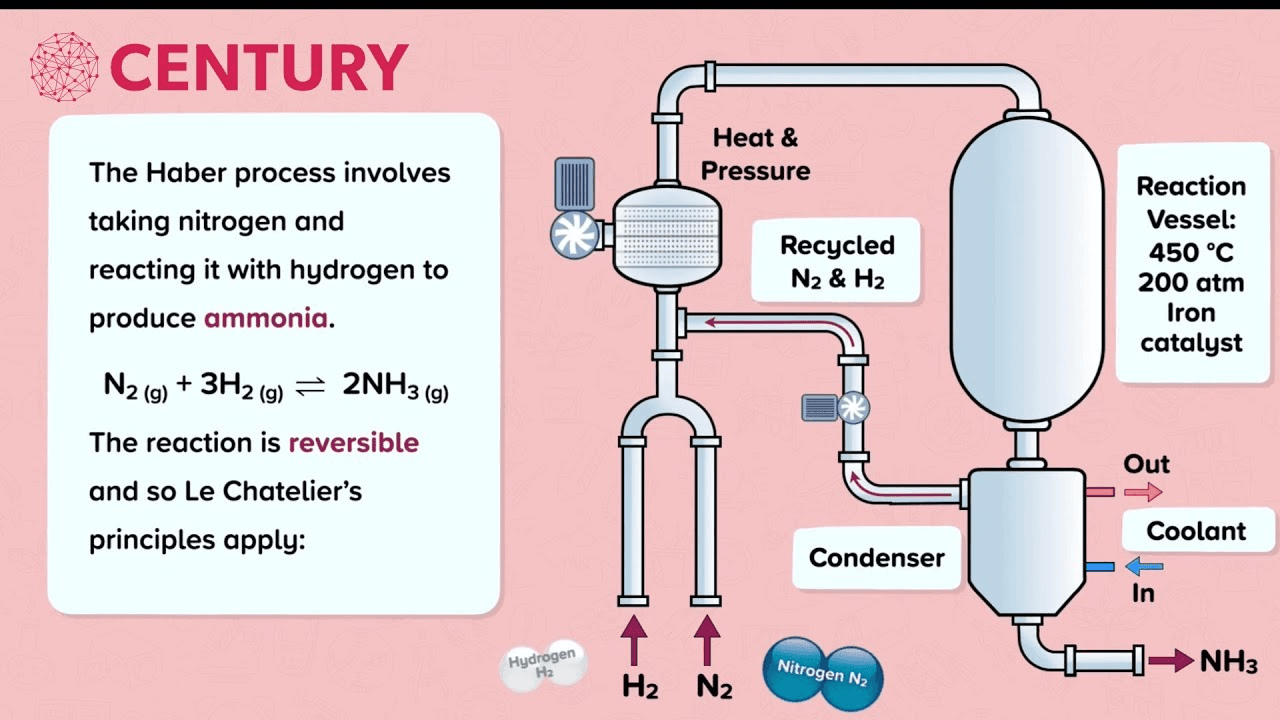
5. Industrial Processes
- Haber Process:
- Key Conditions: 450°C, 200 atm, Iron catalyst.
- Purpose: Synthesis of ammonia for fertilizers.
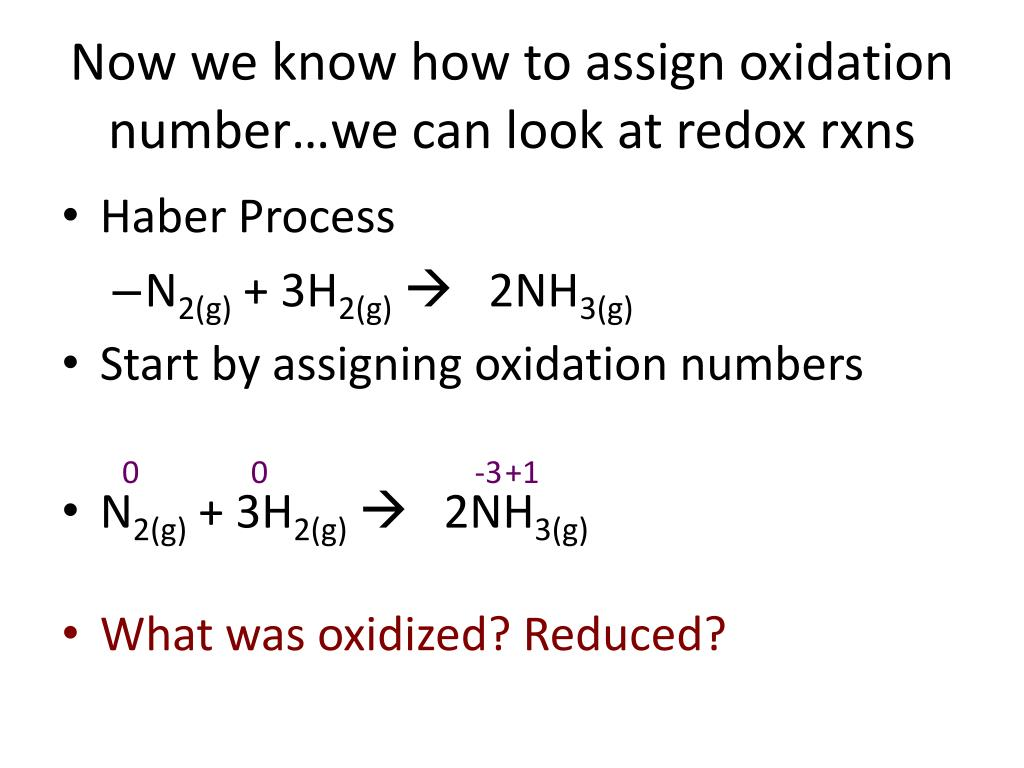
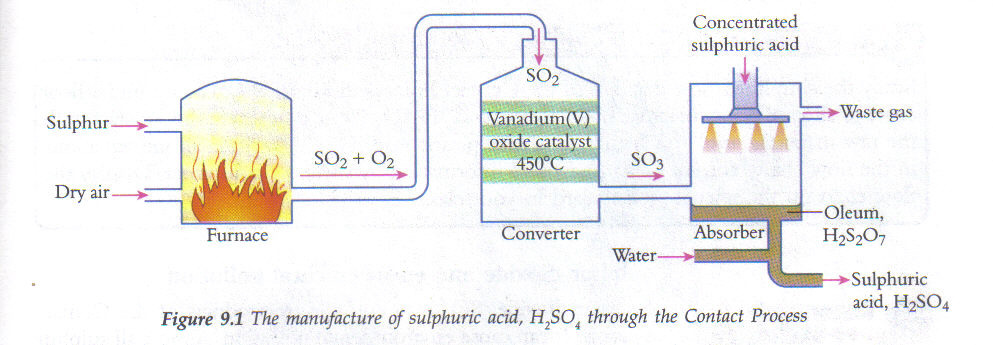
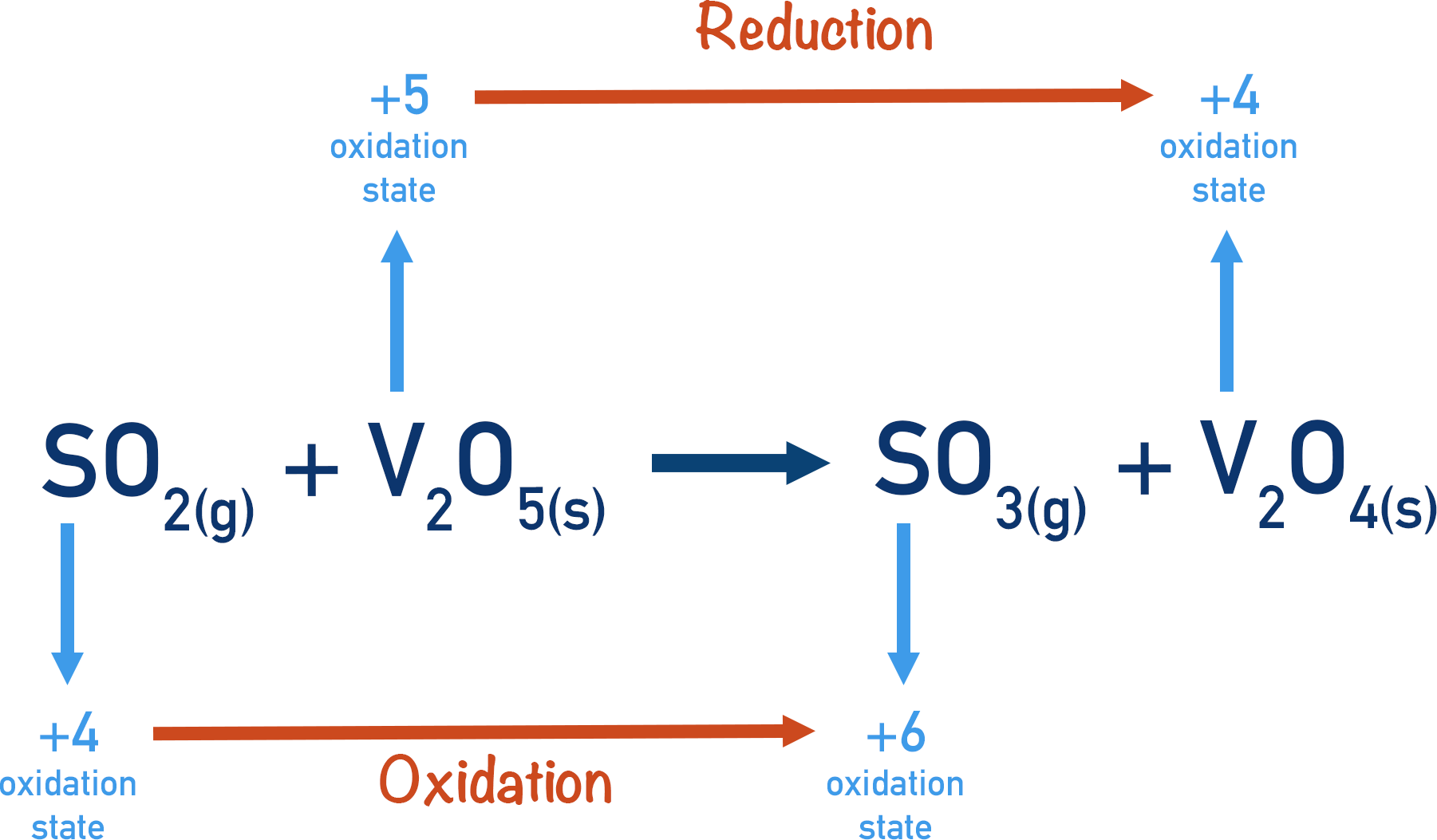
- Contact Process:
- Key Conditions: 450°C, 2 atm, V₂O₅ catalyst.
- Purpose: Production of sulfuric acid.
6. Worked Examples
6.1 Collision Theory Example
- Question: Explain how increasing temperature affects the rate of reaction.
- Answer:
- Explanation: Higher temperature increases the kinetic energy of particles, resulting in more frequent and energetic collisions. This leads to a higher proportion of collisions exceeding the activation energy, thus increasing the reaction rate.
6.2 Redox Reaction Identification
- Question: In the reaction Fe + Cu²⁺ → Fe²⁺ + Cu, identify the oxidizing and reducing agents.
- Answer:
- Fe: Loses electrons (oxidized) → Reducing Agent.
- Cu²⁺: Gains electrons (reduced) → Oxidizing Agent.
6.3 Le Chatelier’s Principle Application
- Question: For the reaction 2NO₂ ⇌ N₂O₄, what happens when pressure is increased?
- Answer:
- Effect: Increasing pressure favors the side with fewer gas molecules (N₂O₄).
- Observation: Mixture becomes more colorless as more N₂O₄ is formed.
6.4 Redox Reaction Example
- Question: Identify which species is acting as the reducing agent in the reaction Fe + Br₂ → FeBr₂.
- Answer:
- Fe: Loses electrons (oxidized) → Reducing Agent.
- Br₂: Gains electrons (reduced) → Oxidizing Agent.
7. Key Concepts and Formulas
7.1 Chemical Equations for Reversible Reactions
- Symbol: ⇌ (double arrow with harpoons).
- Example: N₂ + 3H₂ ⇌ 2NH₃.
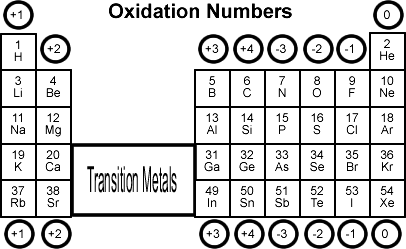
7.2 Oxidation Number Rules
- Element in pure form: 0.
- Monatomic ions: Equal to charge.
- Oxygen: Usually -2.
- Hydrogen: +1 with non-metals, -1 with metals.
- Fluorine: Always -1.
- Sum in compound: Equals overall charge.
7.3 Le Chatelier’s Principle Responses
- Increase in Reactant Concentration: Shift to the right (more products).
- Increase in Product Concentration: Shift to the left (more reactants).
- Increase in Pressure (gases): Shift toward fewer gas molecules.
- Increase in Temperature: Shift toward endothermic direction.
Quizzes
Quiz 1
1. What defines a physical change in a chemical system?
2. Which of the following is an example of a physical change?
3. What characteristic is typical of a chemical change?
4. Which of the following indicators suggests a chemical change has occurred?
5. Which statement correctly differentiates between physical and chemical changes?
6. Which of the following is NOT a method to measure reaction rates?
7. What factor does NOT affect the rate of a chemical reaction?
8. According to the Collision Theory, which condition is necessary for a reaction to occur?
9. What is the purpose of a catalyst in a chemical reaction?
10. Which method is best suited for measuring the rate of a reaction that produces a large volume of gas?
Quiz 2
1. Which of the following best defines a physical change?
2. Which of the following is an example of a physical change?
3. What characteristic is typical of a chemical change?
4. Which of the following indicators suggests a chemical change has occurred?
5. Which statement correctly differentiates between physical and chemical changes?
6. Which of the following is NOT a method to measure reaction rates?
7. What factor does NOT affect the rate of a chemical reaction?
8. According to the Collision Theory, which condition is necessary for a reaction to occur?
9. What is the purpose of a catalyst in a chemical reaction?
10. Which method is best suited for measuring the rate of a reaction that produces a large volume of gas?