07.00 Chapter Summary
BioCast:
1. Physical and Chemical Changes
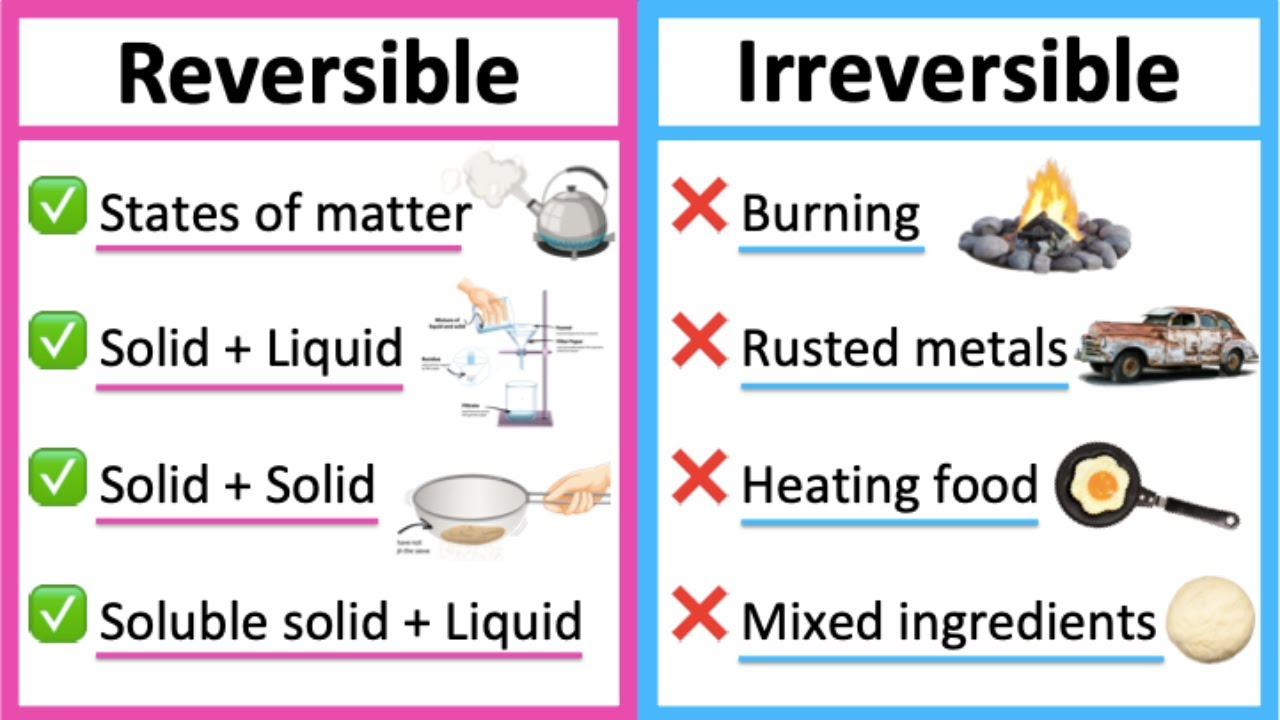
1.1 Physical Changes
- Definition: Changes that do not form new chemical substances.
- Characteristics:
- Reversible: Often can be undone (e.g., melting/freezing).
- Separable: Components can be easily separated using physical methods.
- Common Features:
- No New Substances Formed: Original materials retain their chemical identity.
- Energy Changes: Typically involve changes in state or form without altering chemical bonds.
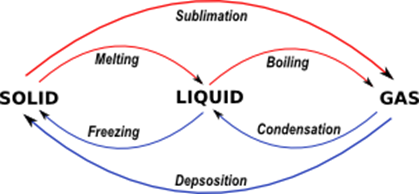
- Examples:
- Changing State:
- Melting: Solid ↔ Liquid (e.g., ice melting to water).
- Evaporation: Liquid ↔ Gas (e.g., water evaporating).
- Sublimation: Solid ↔ Gas (e.g., dry ice sublimating to CO₂ gas).
- Making a Mixture: Combining two or more substances without chemical bonding (e.g., sand and salt mixed together).
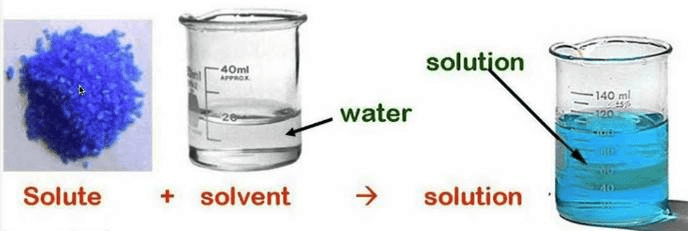
- Dissolving a Solute in a Solvent:
- Example: Sugar dissolving in water to form a sugar solution.
- Changing State:
1.2 Chemical Changes
- Definition: Changes that produce new substances with different properties from the reactants.
- Characteristics:
- Often Irreversible: Difficult to revert to original substances without another chemical reaction.
- Energy Changes: Can be exothermic (release heat) or endothermic (absorb heat).
- Indicators of Chemical Changes:
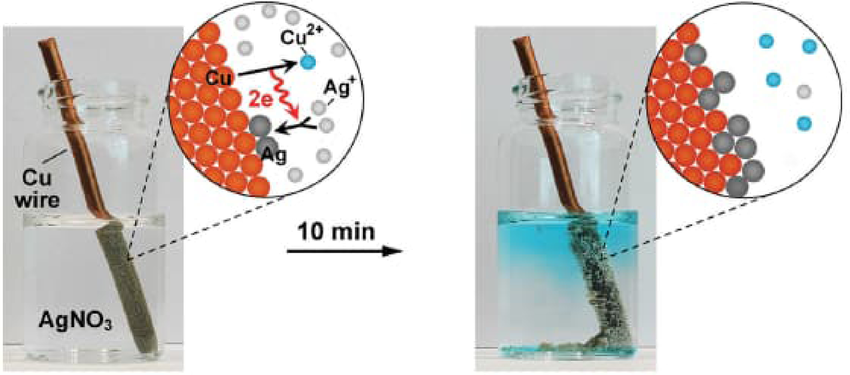
- Color Change:
- Example: Copper displacing silver in a metal displacement reaction:
- Reaction: Cu (orange-brown) + AgNO₃ (colorless) → Cu(NO₃)₂ (blue) + Ag (silver).
- Observation: Solution changes from colorless to blue, and solid changes from orange-brown to silver.
- Example: Copper displacing silver in a metal displacement reaction:
- Temperature Change:
- Exothermic: Releases heat (e.g., reaction of calcium oxide with water).
- Endothermic: Absorbs heat (e.g., dissolution of ammonium chloride in water).
- Effervescence (Fizzing): Release of gas (e.g., reaction of alkali metals with water).
- Formation of a Precipitate: Solid forms from a solution (e.g., halide ion tests forming cream, white, or yellow precipitates).
- Color Change:


2. Introduction to Energy in Chemical Reactions
- Chemical Reactions: Processes where elements achieve a more stable energy state by gaining a full outer shell of electrons through chemical bonding.
- Energy Transfer: Involves the transfer of thermal energy into or out of the reaction mixture, facilitating bond breaking and forming.
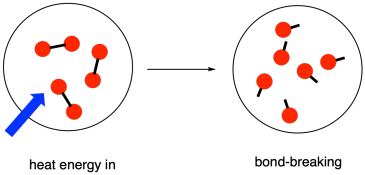
3. Key Terminology
- System: The chemicals undergoing reaction.
- Surroundings: Everything outside the reacting chemicals.
- Chemical Bonds: Act as tiny stores of chemical energy within the system.
4. Heat Exchange in Reactions
- Exothermic Reactions: Release thermal energy from the system to the surroundings.
- Endothermic Reactions: Absorb thermal energy from the surroundings into the system.
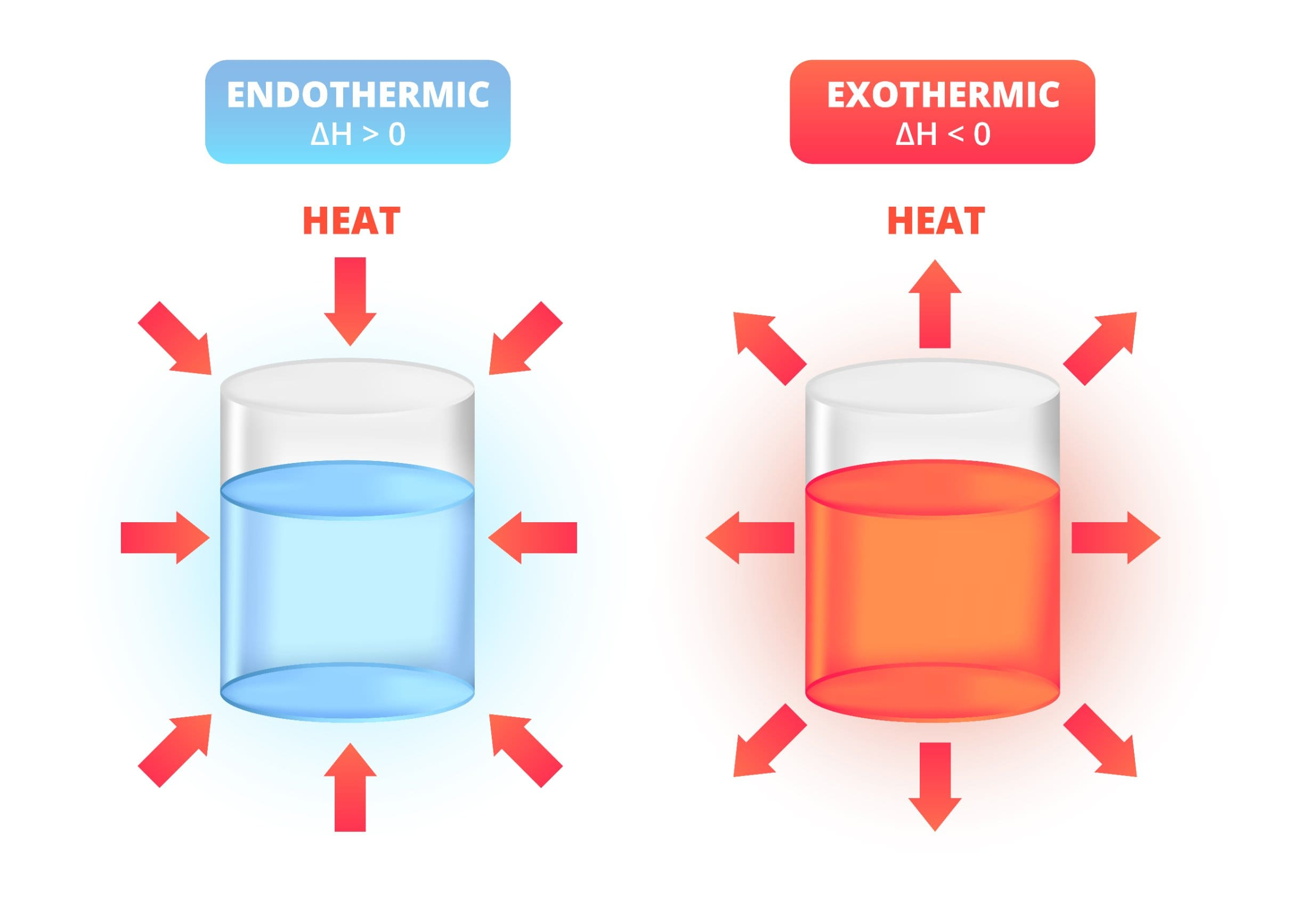
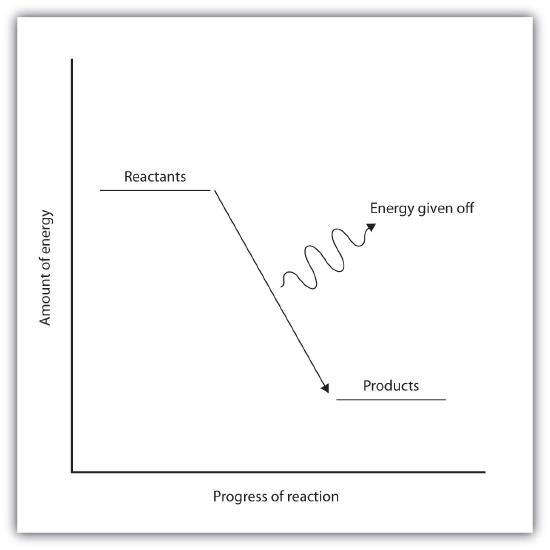
5. Exothermic Reactions
- Definition: Reactions that transfer thermal energy from the chemical system to the surroundings.
- Energy Change (ΔH): Negative (ΔH < 0).
- Temperature Effect: Surroundings become warmer as thermal energy is released.
- Energy Transfer: System → Surroundings.
Examples:
- Combustion: Burning of fuels like methane or gasoline.
- Oxidation: Rusting of iron.
- Neutralization: Reaction between acids and bases (e.g., HCl + NaOH → NaCl + H₂O).
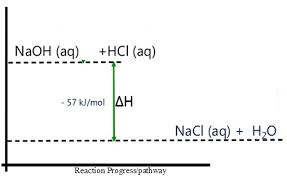
Practical Applications:
- Hand Warmers: Utilize exothermic reactions to release heat and keep hands warm.
- Self-Heating Cans: Containers for food and drinks (e.g., coffee, hot chocolate) that use exothermic reactions to heat their contents.
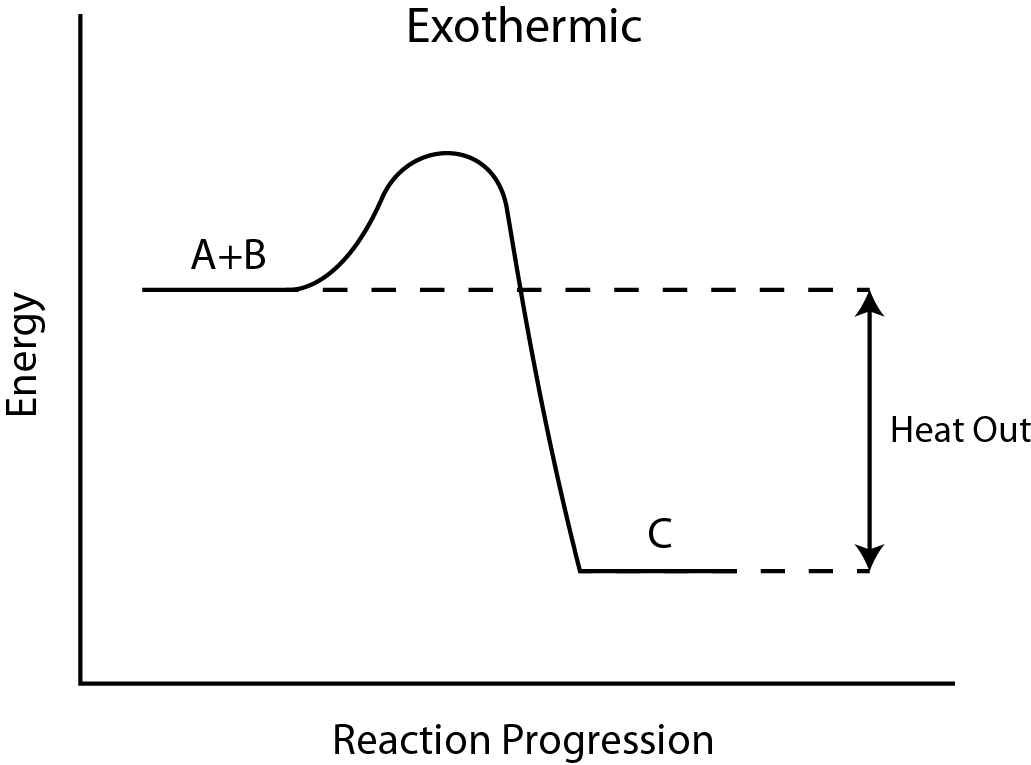
Visual Representation:
- Reaction Pathway Diagram: Products have lower energy than reactants, indicated by a downward arrow.
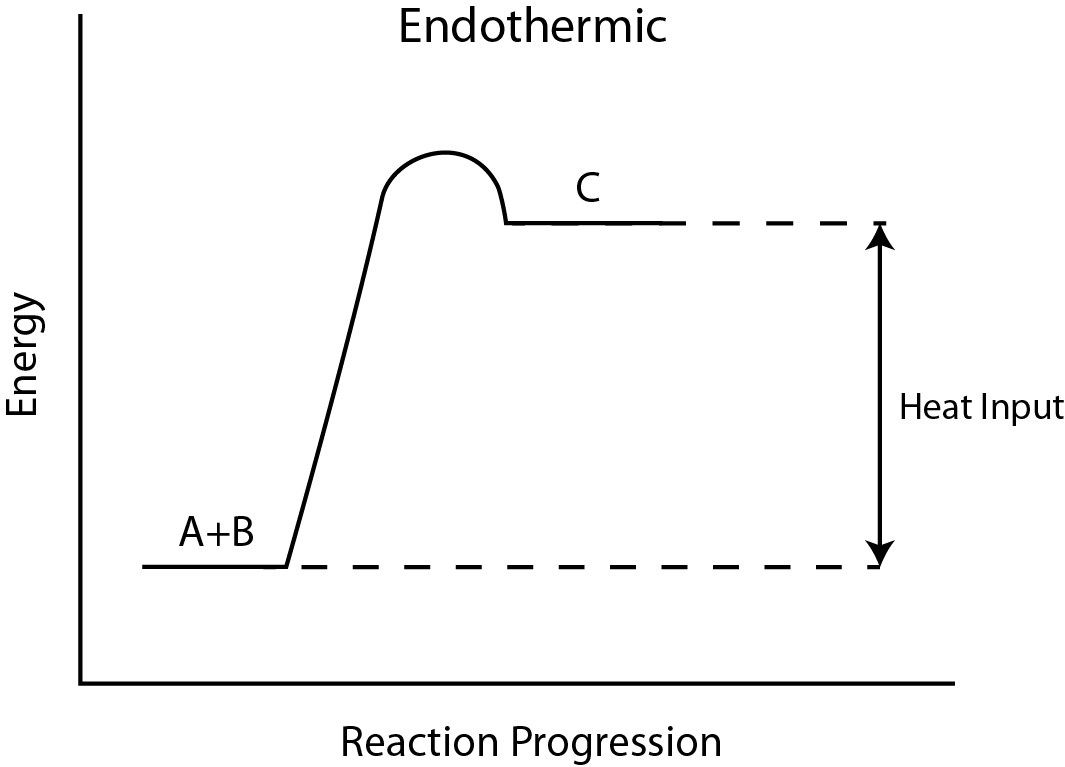
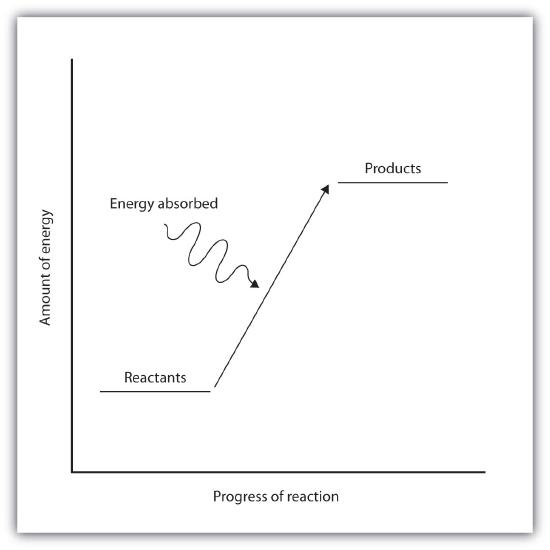
6. Endothermic Reactions
- Definition: Reactions that absorb thermal energy from the surroundings into the system.
- Energy Change (ΔH): Positive (ΔH > 0).
- Temperature Effect: Surroundings become cooler as thermal energy is absorbed.
- Energy Transfer: Surroundings → System.
Examples:
- Electrolysis: Decomposition of water into hydrogen and oxygen using electrical energy.
- Thermal Decomposition: Breaking down calcium carbonate into calcium oxide and carbon dioxide when heated.
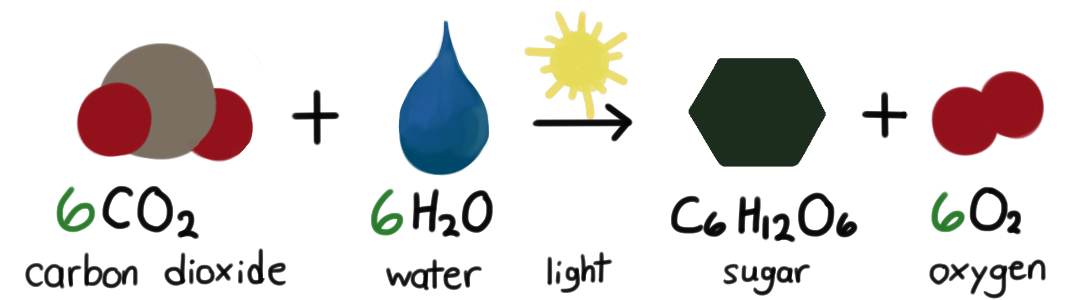
- Photosynthesis: Initial stages where plants absorb energy from sunlight to convert CO₂ and H₂O into glucose and O₂.
Practical Applications:
- Cold Packs: Use endothermic reactions to absorb heat and reduce swelling in sports injuries.
Visual Representation:
- Reaction Pathway Diagram: Products have higher energy than reactants, indicated by an upward arrow.
7. Reaction Pathway Diagrams
- Axes:
- X-axis: Progress of the reaction.
- Y-axis: Energy level.
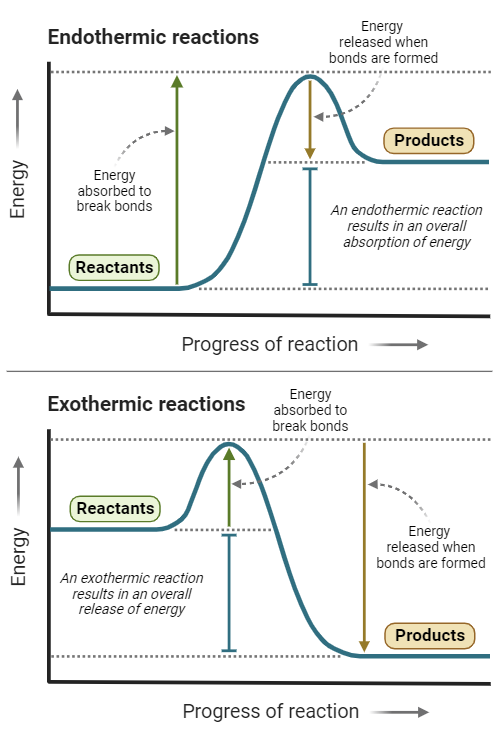
Key Features:
- Reactants and Products: Positioned at different energy levels.
- Enthalpy Change (ΔH): Difference in energy between reactants and products.
- Activation Energy (Ea): Minimum energy required to initiate the reaction.
8. Bond Breaking and Bond Forming
- Bond Breaking: Always endothermic; requires energy input from the surroundings.
- Bond Forming: Always exothermic; releases energy to the surroundings.
- Overall Reaction Energy: Determined by the balance between energy absorbed in breaking bonds and energy released in forming new bonds.
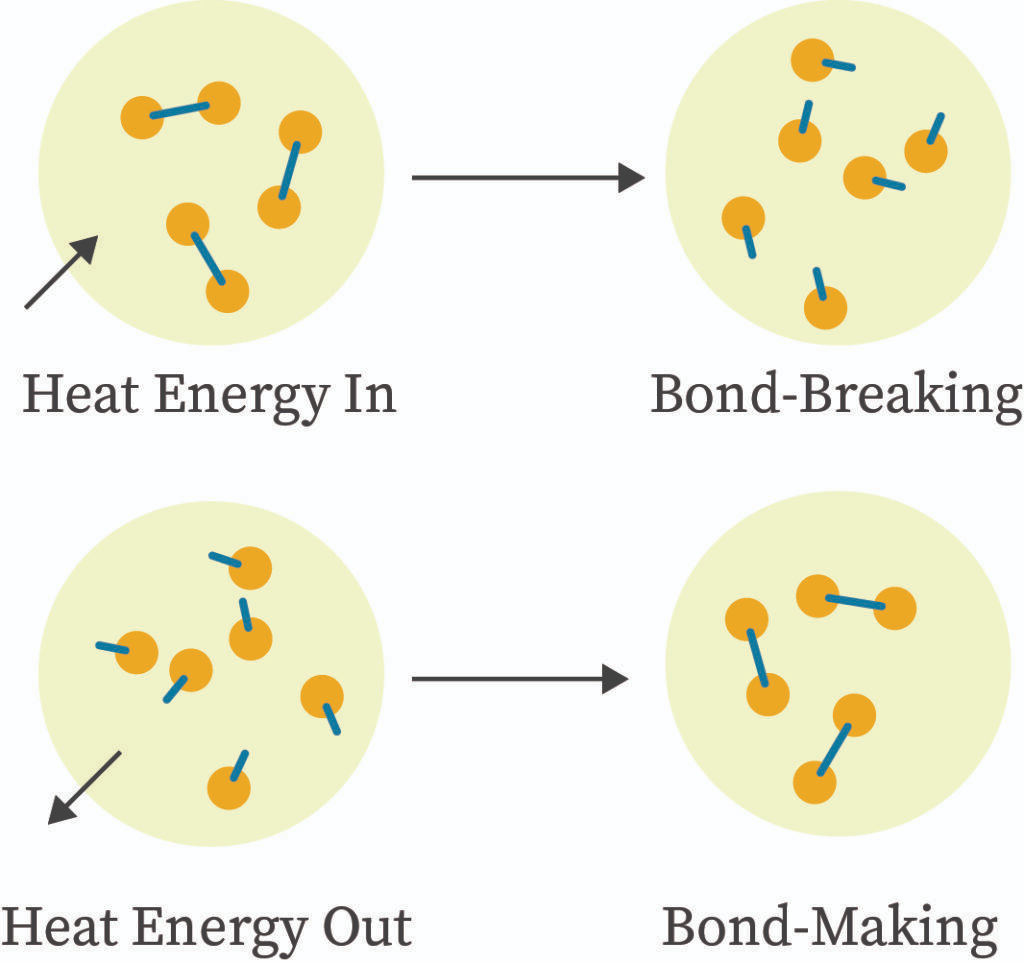
Classification:
- Exothermic Reaction: More energy released in bond formation than absorbed in bond breaking (ΔH < 0).
- Endothermic Reaction: More energy absorbed in bond breaking than released in bond formation (ΔH > 0).
9. Enthalpy Change (ΔH) and Activation Energy (Ea)
- Enthalpy Change (ΔH):
- Negative (ΔH < 0): Exothermic reaction.
- Positive (ΔH > 0): Endothermic reaction.
- Activation Energy (Ea): The minimum energy required for reactants to collide successfully and form products.
Important Notes:
- Different Reactions: Have different activation energies based on the chemical identities involved.
- Higher Activation Energy: Requires more energy to start the reaction.
- Lower Activation Energy: Requires less energy to start the reaction.
10. Bond Energy Calculations
Definition:
- Bond Energy: The amount of energy required to break a bond or the energy released when a bond is formed.
Steps to Calculate ΔH:
- Write a Balanced Equation: Ensure stoichiometry is correct.
- Identify Bonds:
- Reactants: Count all bonds broken.
- Products: Count all bonds formed.
- Calculate Total Bond Energies:
- Energy In: Sum of bond energies for bonds broken (endothermic).
- Energy Out: Sum of bond energies for bonds formed (exothermic).
- Determine ΔH: ΔH = Energy In − Energy Out
- Negative ΔH: Exothermic.
- Positive ΔH: Endothermic.
11. Worked Example
Example 1: Hydrogen and Chlorine Reaction
Reaction:
H2 + Cl2 → 2HCl
Bond Energies:
- H–H: 436 kJ/mol
- Cl–Cl: 242 kJ/mol
- H–Cl: 431 kJ/mol
Calculations:
- Energy In:
H–H + Cl–Cl = 436 + 242 = 678 kJ - Energy Out:
2 × H–Cl = 2 × 431 = 862 kJ - ΔH:
678 – 862 = -184 kJ → Exothermic
- Explanation: Since ΔH is negative, energy is released to the surroundings, making the reaction exothermic.
Example 2: Hydrogen and Iodine Reaction
Reaction:
H2 + I2 → 2HI
Bond Energies:
- H–H: 436 kJ/mol
- I–I: 151 kJ/mol
- H–I: 295 kJ/mol
Calculations:
- Energy In:
H–H + I–I = 436 + 151 = 587 kJ - Energy Out:
2 × H–I = 2 × 295 = 590 kJ - ΔH:
587 – 590 = -3 kJ → Exothermic
- Explanation: Since ΔH is negative, energy is released to the surroundings, making the reaction exothermic.
Example 3: Hydrogen Bromide Decomposition
Reaction:
2HBr → H2 + Br2
Given: ΔH = +103 kJ
Bond Energies:
- H–Br: 366 kJ/mol
- H–H: 436 kJ/mol
- Br–Br: ?
Calculations:
- Energy In:
2 × H–Br = 2 × 366 = 732 kJ - Energy Out:
H–H + Br–Br = 436 + Br–Br - ΔH:
732 – (436 + Br–Br) = +103 kJ
Br–Br=732−436−103=193 kJ/mol
- Explanation: The positive ΔH indicates that the reaction absorbs energy from the surroundings, making it endothermic.
Example 4:
Reaction:
N2 + 3H2 → 2NH3
Bond Energies:
- N≡N: 945 kJ/mol
- H–H: 436 kJ/mol
- N–H: 391 kJ/mol
Calculations:
- Energy In:
N≡N + 3 × H–H = 945 + 3 × 436 = 945 + 1308 = 2253 kJ - Energy Out:
6 × N–H = 6 × 391 = 2346 kJ - ΔH:
2253 – 2346 = -93 kJ → Exothermic
- Explanation: The negative ΔH signifies that energy is released to the surroundings, classifying the reaction as exothermic.
12. Exam Advice:
- Memory Aids:
- EXothermic: Heat EXits the system.
- ENdothermic: Heat ENters the system.
- Temperature Indicators:
- Exothermic: Reaction feels hot.
- Endothermic: Reaction feels cold.
- Diagram Skills:
- Core Candidates: Interpret reaction pathway diagrams.
- Extended Candidates: Draw and interpret reaction pathway diagrams, labeling:
- Reactants
- Products
- Enthalpy Change (ΔH)
- Activation Energy (Ea)
- Bond Energy Calculations:
- Always write a displayed formula to identify bonds.
- Double-check bond counts and energies to avoid mistakes.
Quizzes
Quiz 1
Quiz 2
Quiz 3
Quiz 4
Practice Questions 1
Question 1: Multiple Choice (2 marks)
Which of the following best defines an exothermic reaction?
A) A reaction that absorbs thermal energy from the surroundings.
B) A reaction that releases thermal energy to the surroundings.
C) A reaction where bond breaking requires more energy than bond forming releases.
D) A reaction that does not involve any energy change.
Answer: B) A reaction that releases thermal energy to the surroundings.
Marking Scheme:
- 1 mark for selecting option B.
- 0 marks for any other option.
Explanation:
Exothermic reactions transfer thermal energy from the chemical system to the surroundings, resulting in a temperature increase in the surroundings.
Question 2: Short Answer (3 marks)
Define “activation energy” in the context of chemical reactions.
Answer:
Activation energy (Ea) is the minimum amount of energy required for reactants to collide successfully and form products.
Marking Scheme:
- 1 mark for mentioning it is the minimum energy required.
- 1 mark for specifying it’s for reactant collisions.
- 1 mark for linking it to product formation.
Explanation:
Activation energy is crucial as it determines the rate at which a reaction proceeds. Higher activation energy means the reaction requires more energy to start.
Question 3: Calculation-Based (5 marks)
Calculate the enthalpy change (ΔH) for the following reaction using bond energies:
N2+3H2→2NH3
Bond Energies:
- N≡N: 945 kJ/mol
- H–H: 436 kJ/mol
- N–H: 391 kJ/mol
Answer:
Calculations:
- Energy In (Bonds Broken):
- N≡N: 945 kJ/mol
- 3 H–H: 3 × 436 = 1308 kJ
- Total Energy In: 945 + 1308 = 2253 kJ
- Energy Out (Bonds Formed):
- 6 N–H bonds: 6 × 391 = 2346 kJ
- ΔH = Energy In − Energy Out = 2253 − 2346 = -93 kJ
ΔH = -93 kJ → Exothermic
Marking Scheme:
- 1 mark for correctly calculating total Energy In.
- 1 mark for correctly calculating total Energy Out.
- 1 mark for correctly applying ΔH formula.
- 1 mark for correctly determining the sign of ΔH.
- 1 mark for correctly classifying the reaction as exothermic.
Explanation:
A negative ΔH indicates that energy is released to the surroundings, making the reaction exothermic.
Question 4: True/False (2 marks)
Bond breaking in a chemical reaction is always exothermic.
Answer: False.
Marking Scheme:
- 2 marks for stating “False” with a brief explanation.
- 1 mark if only “False” is stated.
Explanation:
Bond breaking is always endothermic as it requires energy input to break bonds.
Question 5: Diagram Interpretation (4 marks)
Refer to the Reaction Pathway Diagram below.
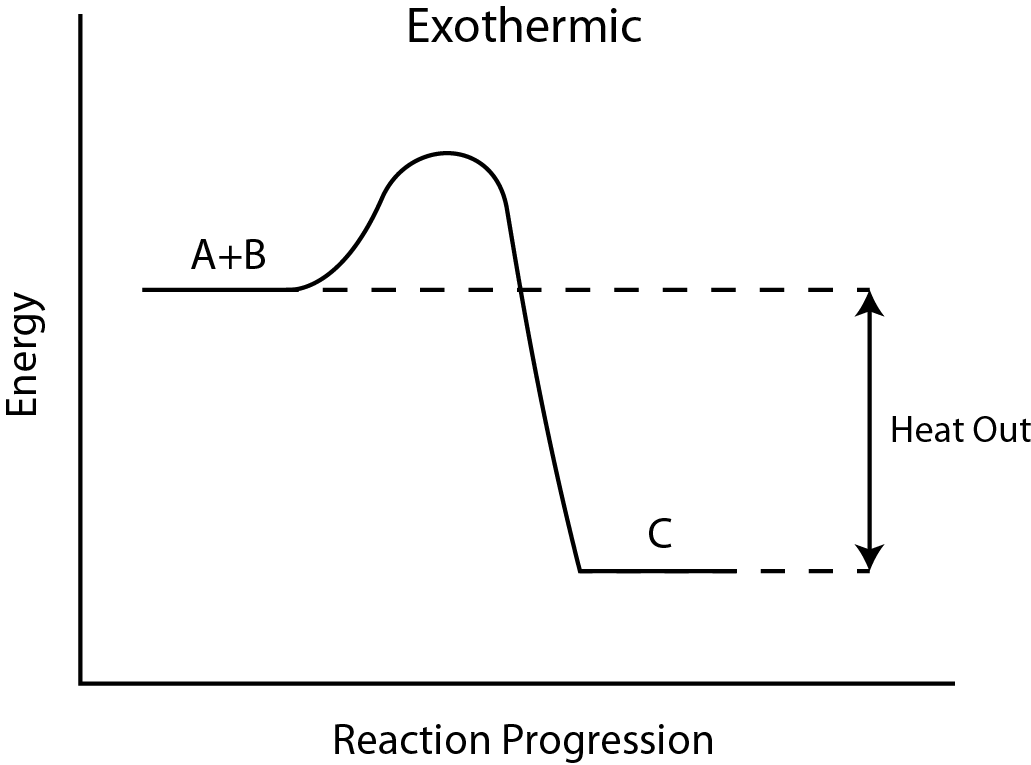
Based on the diagram, answer the following:
a) Is the reaction exothermic or endothermic?
b) Which direction does the energy transfer?
Answer:
a) Exothermic.
b) From the system to the surroundings.
Marking Scheme:
- a) 2 marks:
- 1 mark for identifying as exothermic.
- 1 mark for correct reasoning based on energy levels.
- b) 2 marks:
- 1 mark for identifying the correct direction (system to surroundings).
- 1 mark for linking it to the exothermic nature.
Question 6: Bond Energy Calculation (5 marks)
Given the reaction:
H2+Cl2→2HCl
Bond Energies:
- H–H: 436 kJ/mol
- Cl–Cl: 242 kJ/mol
- H–Cl: 431 kJ/mol
Calculate ΔH for the reaction.
Answer:
Calculations:
- Energy In:
- H–H: 436 kJ
- Cl–Cl: 242 kJ
- Total Energy In: 436 + 242 = 678 kJ
- Energy Out:
- 2 H–Cl: 2 × 431 = 862 kJ
- ΔH = Energy In − Energy Out = 678 − 862 = -184 kJ
ΔH = -184 kJ → Exothermic
Marking Scheme:
- 1 mark for calculating Energy In correctly.
- 1 mark for calculating Energy Out correctly.
- 1 mark for applying ΔH formula correctly.
- 1 mark for determining the sign of ΔH.
- 1 mark for correctly classifying the reaction as exothermic.
Explanation:
A negative ΔH indicates energy is released, confirming the reaction is exothermic.
Question 7: Short Answer (3 marks)
Explain the difference between bond breaking and bond forming in terms of energy exchange.
Answer:
Bond breaking is always endothermic, requiring energy input to break bonds. Bond forming is always exothermic, releasing energy when new bonds are formed.
Marking Scheme:
- 1 mark for stating bond breaking is endothermic.
- 1 mark for stating bond forming is exothermic.
- 1 mark for explaining energy input and release respectively.
Question 8: Application-Based (4 marks)
Describe a practical application of an endothermic reaction and explain how it works.
Answer:
Example: Cold packs used in sports injuries.
Explanation: These packs contain chemicals that undergo an endothermic reaction when activated, absorbing heat from the surroundings and providing a cooling effect to reduce swelling.
Marking Scheme:
- 2 marks for correctly identifying a practical application (e.g., cold packs).
- 2 marks for explaining how the endothermic reaction works in that context.
Explanation:
Endothermic reactions absorb heat, making them ideal for applications requiring cooling, such as reducing inflammation in injuries.
Question 9: Comparison (3 marks)
Compare exothermic and endothermic reactions in terms of ΔH and temperature effect on the surroundings.
Answer:
- Exothermic Reactions:
- ΔH is negative (ΔH < 0).
- Surroundings become warmer as energy is released.
- Endothermic Reactions:
- ΔH is positive (ΔH > 0).
- Surroundings become cooler as energy is absorbed.
Marking Scheme:
- 1 mark for correct ΔH for exothermic.
- 1 mark for correct temperature effect for exothermic.
- 1 mark for correct ΔH and temperature effect for endothermic.
Explanation:
Exothermic reactions release energy, lowering the system’s energy and increasing the surroundings’ temperature. Endothermic reactions absorb energy, increasing the system’s energy and decreasing the surroundings’ temperature.
Question 10: Diagram Drawing (5 marks)
Draw a reaction pathway diagram for an endothermic reaction, labeling the reactants, products, ΔH, and activation energy (Ea).
Answer:
A correctly drawn diagram should include:
- Reactants positioned at a lower energy level.
- Products positioned at a higher energy level.
- Upward arrow indicating the endothermic nature.
- ΔH labeled as positive.
- Activation Energy (Ea) shown as a peak between reactants and products.
Marking Scheme:
- 1 mark for correctly positioning reactants and products.
- 1 mark for indicating higher energy of products.
- 1 mark for showing ΔH as positive.
- 1 mark for depicting activation energy as a peak.
- 1 mark for proper labeling of all parts.
Explanation:
In an endothermic reaction pathway diagram, products have higher energy than reactants, indicating energy absorption. The activation energy is the peak that reactants must overcome to form products.
Question 11: Multiple Choice (2 marks)
Which statement correctly describes an endothermic reaction?
A) It releases energy, causing the surroundings to heat up.
B) It absorbs energy, causing the surroundings to cool down.
C) It does not involve any energy transfer.
D) It always has a negative ΔH.
Answer: B) It absorbs energy, causing the surroundings to cool down.
Marking Scheme:
- 2 marks for selecting option B.
- 0 marks for any other option.
Explanation:
Endothermic reactions absorb thermal energy from the surroundings, leading to a cooling effect in the surrounding environment.
Question 12: Short Answer (3 marks)
What is the relationship between bond energy and the stability of a chemical bond?
Answer:
Higher bond energy indicates a stronger and more stable chemical bond, as more energy is required to break it. Conversely, lower bond energy signifies a weaker bond that is easier to break.
Marking Scheme:
- 1 mark for stating that higher bond energy relates to stronger bonds.
- 1 mark for mentioning that lower bond energy relates to weaker bonds.
- 1 mark for explaining the implication on bond stability.
Explanation:
Understanding bond energy helps predict the strength and stability of chemical bonds, which is crucial in determining the energy changes during reactions.
Question 13: Calculation-Based (5 marks)
Calculate the enthalpy change (ΔH) for the following reaction using bond energies:
2NO2→N2O4
Bond Energies:
- N=O double bond: 607 kJ/mol
- N–N single bond: 163 kJ/mol
- O=O double bond: 498 kJ/mol
Answer:
Balanced Reaction:
2NO2→N2O4
Bond Breaking and Forming:
- Bonds Broken:
- 2 × N=O (in 2NO₂) = 2 × 607 = 1214 kJ
- Bonds Formed:
- N–N (in N₂O₄) = 163 kJ
- Additionally, in N₂O₄, each NO₂ unit forms an additional O–O bond (assuming structure): 2 × O=O = 2 × 498 = 996 kJ
- Total Bonds Formed: 163 + 996 = 1159 kJ
ΔH = Energy In − Energy Out = 1214 − 1159 = +55 kJ
ΔH = +55 kJ → Endothermic
Marking Scheme:
- 1 mark for correctly balancing the equation.
- 1 mark for calculating total energy of bonds broken.
- 1 mark for calculating total energy of bonds formed.
- 1 mark for correctly applying ΔH formula.
- 1 mark for correctly classifying the reaction as endothermic.
Explanation:
A positive ΔH indicates that the reaction absorbs energy from the surroundings, making it endothermic.
Question 14: True/False (2 marks)
In an exothermic reaction, the activation energy (Ea) is always lower than in an endothermic reaction.
Answer: False.
Marking Scheme:
- 2 marks for stating “False” with a correct brief explanation.
- 1 mark if only “False” is stated.
Explanation:
Activation energy depends on the specific reaction and not solely on whether it is exothermic or endothermic. Some exothermic reactions may have higher activation energies than certain endothermic reactions.
Question 15: Diagram Interpretation (4 marks)
Refer to the Reaction Pathway Diagram below.

Based on the diagram, answer the following:
a) Is the reaction exothermic or endothermic?
b) What does the activation energy represent in this diagram?
Answer:
a) Endothermic.
b) Activation energy represents the minimum energy required for the reactants to transform into products.
Marking Scheme:
- a) 2 marks:
- 1 mark for identifying as endothermic.
- 1 mark for correct reasoning based on energy levels.
- b) 2 marks:
- 1 mark for defining activation energy as the energy barrier.
- 1 mark for linking it to the transformation from reactants to products.
Explanation:
An upward arrow indicates that products have higher energy than reactants, signifying an endothermic reaction. Activation energy is the peak that reactants must overcome to form products.
Question 16: Bond Energy Calculation (5 marks)
Given the reaction:
CaCO3→CaO+CO2
Bond Energies:
- Ca–O: 350 kJ/mol
- C–O: 534 kJ/mol
- O=C=O (in CO₂): 799 kJ/mol
Calculate ΔH for the reaction.
Answer:
Balanced Reaction:
CaCO3→CaO+CO2
Bonds Broken and Formed:
- Bonds Broken:
- Ca–O bonds in CaCO₃: Assuming 3 Ca–O bonds = 3 × 350 = 1050 kJ
- C–O bonds in CaCO₃: Assuming 3 C–O bonds = 3 × 534 = 1602 kJ
- Total Energy In: 1050 + 1602 = 2652 kJ
- Bonds Formed:
- Ca–O bond in CaO: 350 kJ
- O=C=O bonds in CO₂: 2 × 799 = 1598 kJ
- Total Energy Out: 350 + 1598 = 1948 kJ
ΔH = Energy In − Energy Out = 2652 − 1948 = +704 kJ
ΔH = +704 kJ → Endothermic
Marking Scheme:
- 1 mark for correctly identifying and calculating bonds broken.
- 1 mark for correctly identifying and calculating bonds formed.
- 1 mark for correctly applying the ΔH formula.
- 1 mark for determining the sign of ΔH.
- 1 mark for correctly classifying the reaction as endothermic.
Explanation:
The positive ΔH indicates that the reaction absorbs energy, confirming it is endothermic.
Question 17: Short Answer (3 marks)
Describe how self-heating cans utilize exothermic reactions to function.
Answer:
Self-heating cans contain chemicals that undergo exothermic reactions when activated, releasing thermal energy. This heat warms the contents of the can, allowing food or beverages to be heated without external heat sources.
Marking Scheme:
- 1 mark for identifying the use of exothermic reactions.
- 1 mark for explaining the release of thermal energy.
- 1 mark for linking the energy release to heating the can’s contents.
Explanation:
Exothermic reactions provide a convenient and portable means of generating heat, making self-heating cans practical for on-the-go food and drink preparation.
Question 18: Application-Based (4 marks)
Explain how photosynthesis is an example of an endothermic reaction and its significance in nature.
Answer:
Photosynthesis absorbs energy from sunlight (endothermic) to convert carbon dioxide and water into glucose and oxygen. This energy storage process is essential for producing the organic compounds that fuel most life on Earth and for generating oxygen necessary for respiration.
Marking Scheme:
- 2 marks for correctly identifying photosynthesis as an endothermic reaction.
- 2 marks for explaining the absorption of sunlight energy and its significance in energy storage and oxygen production.
Explanation:
Photosynthesis is fundamental to life, as it transforms solar energy into chemical energy, supporting ecosystems and maintaining atmospheric oxygen levels.
Question 19: Comparison (3 marks)
Compare bond breaking and bond forming in terms of their impact on the overall enthalpy change (ΔH) of a reaction.
Answer:
- Bond Breaking: Absorbs energy (endothermic), increasing ΔH.
- Bond Forming: Releases energy (exothermic), decreasing ΔH.
- Overall ΔH: Depends on whether more energy is absorbed in breaking bonds or released in forming bonds.
Marking Scheme:
- 1 mark for explaining bond breaking absorbs energy.
- 1 mark for explaining bond forming releases energy.
- 1 mark for linking these processes to the overall ΔH.
Explanation:
The balance between energy absorbed in breaking bonds and energy released in forming new bonds determines whether a reaction is exothermic or endothermic.
Question 20: Diagram Drawing (5 marks)
Draw a reaction pathway diagram for an exothermic reaction, labeling the reactants, products, ΔH, and activation energy (Ea).
Answer:
A correctly drawn diagram should include:
- Reactants positioned at a higher energy level.
- Products positioned at a lower energy level.
- Downward arrow indicating the exothermic nature.
- ΔH labeled as negative.
- Activation Energy (Ea) shown as a peak between reactants and products.
Marking Scheme:
- 1 mark for correctly positioning reactants and products.
- 1 mark for indicating lower energy of products.
- 1 mark for showing ΔH as negative.
- 1 mark for depicting activation energy as a peak.
- 1 mark for proper labeling of all parts.
Explanation:
In an exothermic reaction pathway diagram, the release of energy is visualized by products having lower energy than reactants, and the activation energy represents the energy barrier that must be overcome to initiate the reaction.
Question 21: Short Answer (3 marks)
What practical application utilizes the endothermic decomposition of hydrogen bromide (HBr) and how?
Answer:
- Example: Cold packs used in medical applications.
Marking Scheme:
- 1 mark for identifying a practical application (e.g., cold packs).
- 2 marks for explaining how the endothermic decomposition absorbs heat to provide cooling.
Explanation: These packs exploit the endothermic decomposition of HBr to absorb heat from the surroundings, providing a cooling effect to reduce inflammation and swelling in injuries.
Question 22: Multiple Choice (2 marks)
Which of the following reactions is endothermic?
A) Combustion of methane.
B) Formation of water from hydrogen and oxygen.
C) Photosynthesis in plants.
D) Neutralization of hydrochloric acid with sodium hydroxide.
Answer: C) Photosynthesis in plants.
Marking Scheme:
- 2 marks for selecting option C.
- 0 marks for any other option.
Explanation:
Photosynthesis absorbs energy from sunlight, making it an endothermic process, whereas the other options involve exothermic reactions that release energy.
Question 23: Calculation-Based (5 marks)
Calculate the bond energy of Br–Br given the decomposition reaction of hydrogen bromide:
2HBr→H2+Br2
Given:
- ΔH = +103 kJ
- H–Br bond energy = 366 kJ/mol
- H–H bond energy = 436 kJ/mol
Answer:
Balanced Reaction:
2HBr→H2+Br2
Bonds Broken and Formed:
- Bonds Broken:
- 2 × H–Br = 2 × 366 = 732 kJ
- Bonds Formed:
- 1 × H–H = 436 kJ
- 1 × Br–Br = X kJ
ΔH = Energy In − Energy Out = 732 − (436 + X) = +103 kJ
Solving for X:
732−436−X=103
732−436=296
296−X=103
296 – X = 1032
96−X=103
X=296−103=193 kJ/mol
Bond Energy of Br–Br = 193 kJ/mol
Marking Scheme:
- 1 mark for identifying bonds broken correctly.
- 1 mark for identifying bonds formed correctly.
- 1 mark for setting up the ΔH equation.
- 1 mark for solving the equation correctly.
- 1 mark for correctly stating the bond energy of Br–Br.
Explanation:
By accounting for the energy required to break bonds and the energy released in forming new bonds, the bond energy of Br–Br is determined to be 193 kJ/mol, indicating an endothermic reaction.
Question 23: Multiple Choice (2 marks)
Which of the following best describes the activation energy (Ea) of a reaction?
A) The total energy released during the reaction.
B) The difference in energy between reactants and products.
C) The energy required to break all bonds in the reactants.
D) The minimum energy required to initiate the reaction.
Answer: D) The minimum energy required to initiate the reaction.
Marking Scheme:
- 2 marks for selecting option D.
- 0 marks for any other option.
Explanation:
Activation energy is the threshold energy that reactants must possess for a reaction to occur. It represents the minimum energy required to start the reaction by overcoming the energy barrier.
Question 24: Short Answer (3 marks)
Explain why the combustion of methane (CH₄) is considered an exothermic reaction.
Answer:
The combustion of methane is exothermic because it releases a significant amount of thermal energy to the surroundings. During the reaction, methane bonds are broken and new bonds in carbon dioxide and water are formed, releasing more energy than is absorbed, resulting in a net release of heat.
Marking Scheme:
- 1 mark for identifying the release of thermal energy.
- 1 mark for mentioning bond breaking and forming.
- 1 mark for explaining that more energy is released than absorbed.
Explanation:
In exothermic reactions like methane combustion, the energy released from forming new bonds exceeds the energy required to break the initial bonds, leading to a net release of heat into the surroundings.
Question 25: Calculation-Based (5 marks)
Calculate the enthalpy change (ΔH) for the following reaction using bond energies:
2H2O→2H2+O2
Bond Energies:
- O–H: 467 kJ/mol
- H–H: 436 kJ/mol
- O=O: 498 kJ/mol
Answer:
Balanced Reaction:
2H2O→2H2+O2
Bonds Broken and Formed:
- Bonds Broken:
- 4 O–H bonds in 2 H₂O: 4 × 467 = 1,868 kJ
- Bonds Formed:
- 2 H–H bonds in 2 H₂: 2 × 436 = 872 kJ
- 1 O=O bond in O₂: 498 kJ
- Total Energy Out: 872 + 498 = 1,370 kJ
- ΔH = Energy In − Energy Out = 1,868 − 1,370 = +498 kJ
ΔH = +498 kJ → Endothermic
Marking Scheme:
- 1 mark for correctly balancing the equation.
- 1 mark for calculating total energy of bonds broken.
- 1 mark for calculating total energy of bonds formed.
- 1 mark for correctly applying the ΔH formula.
- 1 mark for correctly classifying the reaction as endothermic.
Explanation:
A positive ΔH indicates that the reaction absorbs energy from the surroundings, making it endothermic. The energy required to break the O–H bonds exceeds the energy released from forming H–H and O=O bonds.
Question 26: True/False (2 marks)
All endothermic reactions require an external source of energy to proceed.
Answer: True.
Marking Scheme:
- 2 marks for stating “True” with a correct brief explanation.
- 1 mark if only “True” is stated.
Explanation:
Endothermic reactions absorb energy from their surroundings to proceed. Therefore, an external energy source is necessary to provide the required thermal energy for the reaction to occur.
Question 27: Diagram Interpretation (4 marks)
Refer to the Reaction Pathway Diagram below.

Based on the diagram, answer the following:
a) What does the upward arrow signify in the reaction pathway?
b) How does the energy of the products compare to the reactants?
Answer:
a) The upward arrow signifies that the reaction is endothermic, indicating energy absorption.
b) The products have higher energy than the reactants.
Marking Scheme:
- a) 2 marks:
- 1 mark for identifying it signifies an endothermic reaction.
- 1 mark for explaining it indicates energy absorption.
- b) 2 marks:
- 1 mark for stating products have higher energy.
- 1 mark for correct comparison with reactants.
Explanation:
An upward arrow in a reaction pathway diagram indicates that energy is absorbed during the reaction, characteristic of endothermic processes. Additionally, products being at a higher energy level than reactants confirms the endothermic nature of the reaction.
Question 28: Application-Based (4 marks)
Describe how hand warmers utilize exothermic reactions to provide heat.
Answer:
Hand warmers contain chemicals, such as iron powder, salt, water, and activated carbon, that undergo an exothermic oxidation reaction when exposed to air. The oxidation of iron releases thermal energy, which warms the surrounding area, providing heat to keep hands warm.
Marking Scheme:
- 2 marks for correctly identifying the chemicals involved (e.g., iron oxidation).
- 2 marks for explaining the exothermic reaction releases heat to warm the hands.
Explanation:
In hand warmers, the exothermic oxidation of iron provides a steady release of heat, making them effective for keeping hands warm in cold environments by transferring energy to the surroundings.
Question 29: Short Answer (3 marks)
What role does bond energy play in determining whether a reaction is exothermic or endothermic?
Answer:
Bond energy determines the overall energy change in a reaction by quantifying the energy required to break bonds and the energy released when new bonds form. If the total bond energy released in forming products is greater than the energy absorbed in breaking reactant bonds, the reaction is exothermic. Conversely, if more energy is absorbed in breaking bonds than is released in forming new bonds, the reaction is endothermic.
Marking Scheme:
- 1 mark for explaining bond breaking absorbs energy.
- 1 mark for explaining bond forming releases energy.
- 1 mark for linking the balance of these energies to exothermic or endothermic classification.
Explanation:
Understanding bond energies allows prediction of the reaction’s enthalpy change by comparing energy absorbed and released, thus determining if the reaction will release or absorb heat.
Question 30: Calculation-Based (5 marks)
Given the following reaction, calculate the bond energy of the Br–Br bond:
2HBr→H2+Br2
Given:
- ΔH = +103 kJ
- H–Br bond energy = 366 kJ/mol
- H–H bond energy = 436 kJ/mol
Answer:
Balanced Reaction:
2HBr→H2+Br2
Bonds Broken and Formed:
- Bonds Broken:
- 2 × H–Br bonds = 2 × 366 = 732 kJ
- Bonds Formed:
- 1 × H–H bond = 436 kJ
- 1 × Br–Br bond = X kJ
- ΔH = Energy In − Energy Out = 732 − (436 + X) = +103 kJ
Solving for X:
732−436−X=103
296−X=103
X=296−103=193 kJ/mol
Bond Energy of Br–Br = 193 kJ/mol
Marking Scheme:
- 1 mark for correctly balancing the equation.
- 1 mark for calculating total energy of bonds broken.
- 1 mark for setting up the ΔH equation.
- 1 mark for correctly solving the equation for X.
- 1 mark for stating the correct bond energy of Br–Br.
Explanation:
By accounting for the energy required to break the H–Br bonds and the energy released from forming H–H and Br–Br bonds, the bond energy of Br–Br is determined to be 193 kJ/mol, consistent with the endothermic nature of the reaction.
Question 31: True/False (2 marks)
In an endothermic reaction pathway diagram, the activation energy is higher than the overall enthalpy change (ΔH).
Answer: True.
Marking Scheme:
- 2 marks for stating “True” with a correct brief explanation.
- 1 mark if only “True” is stated.
Explanation:
In endothermic reactions, the activation energy must account for both the energy required to reach the transition state and the energy absorbed (ΔH). Therefore, Ea is greater than ΔH.
Question 32: Comparison (3 marks)
Compare the temperature effects on the surroundings for exothermic and endothermic reactions.
Answer:
- Exothermic Reactions: Release heat to the surroundings, causing the surroundings to become warmer.
- Endothermic Reactions: Absorb heat from the surroundings, causing the surroundings to become cooler.
Marking Scheme:
- 1 mark for describing the temperature effect of exothermic reactions.
- 1 mark for describing the temperature effect of endothermic reactions.
- 1 mark for correctly contrasting the two effects.
Explanation:
Exothermic reactions transfer heat outward, increasing the temperature around them, while endothermic reactions draw heat inward, decreasing the temperature of their surroundings.
Question 33: Short Answer (3 marks)
Why is photosynthesis considered an important endothermic reaction in nature?
Answer:
Photosynthesis is important because it absorbs energy from sunlight to convert carbon dioxide and water into glucose and oxygen. This endothermic process is fundamental for producing the organic compounds that sustain life and for generating oxygen necessary for respiration in most living organisms.
Marking Scheme:
- 1 mark for identifying photosynthesis as endothermic.
- 1 mark for explaining energy absorption from sunlight.
- 1 mark for describing its significance in producing glucose and oxygen.
Explanation:
Photosynthesis captures solar energy, storing it in chemical bonds of glucose, which serves as an energy source for living organisms, while also replenishing atmospheric oxygen.
Question 34: Application-Based (4 marks)
Explain how cold packs use endothermic reactions to provide cooling and reduce swelling.
Answer:
Cold packs contain chemicals that, when mixed or activated, undergo an endothermic reaction. For example, ammonium nitrate dissolving in water absorbs heat from the surroundings, including the injured area. This absorption of thermal energy lowers the temperature, providing a cooling effect that helps reduce swelling and numb pain.
Marking Scheme:
- 2 marks for describing the endothermic reaction (e.g., ammonium nitrate dissolution).
- 2 marks for explaining how heat absorption leads to cooling and reduces swelling.
Explanation:
Endothermic reactions in cold packs absorb heat, making them effective for lowering the temperature at injury sites, which helps in managing inflammation and discomfort.