9.03 Fertilisers
1. Introduction to Fertilisers
a. Importance of Fertilisers:
- Role in Agriculture:
- Plants require essential nutrients to grow, develop healthy leaves, roots, flowers, and fruits.
- While plants perform photosynthesis using carbon dioxide and water, they rely on minerals from the soil for additional nutrients.
- Nutrient Depletion:
- Intensive farming depletes soil minerals, necessitating the replenishment of these nutrients through artificial fertilisers.
- Global Food Supply:
- Fertilisers enable faster plant growth and larger crop yields, crucial for feeding the world’s growing population.
b. Types of Fertilisers:
- Straight N Fertilisers:
- Contain only nitrogen (N) compounds.
- Examples: Ammonium nitrate (NH₄NO₃), Ammonium sulfate ((NH₄)₂SO₄), Urea (CO(NH₂)₂).
- Compound Fertilisers (NPK Fertilisers):
- Contain a mixture of nitrogen (N), phosphorus (P), and potassium (K).
- Examples: NPK 21:8:11 (21% N, 8% P, 11% K).
2. Nitrogenous Fertilisers
a. Ammonium Salts:
- Ammonium Nitrate (NH₄NO₃):
- Composition: Contains both NH₄⁺ (ammonium) and NO₃⁻ (nitrate) ions.
- Production Reaction:
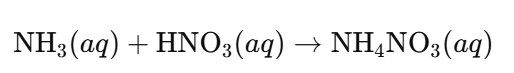
- Properties:
- Solubility: Highly soluble in water, allowing plants to uptake nitrogen easily.
- Mass Composition: Contains 35% nitrogen by mass.
- Uses: Widely used as a nitrogenous fertiliser and in the production of explosives.
- Ammonium Sulfate ((NH₄)₂SO₄):
- Production Reaction:
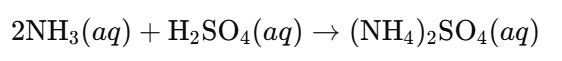
- Properties:
- Solubility: Soluble in water.
- Effect on Soil: Makes soil slightly acidic; often mixed with chalk (calcium carbonate) to neutralize acidity.
- Urea (CO(NH₂)₂):
- Production Reaction:
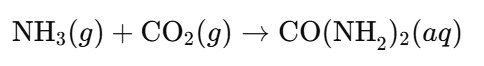
- Properties:
- Solubility: Highly soluble in water.
- Uses: Major nitrogenous fertiliser due to high nitrogen content (46% N).
b. Nitrates:
- Potassium Nitrate (KNO₃):
- Composition: Contains K⁺ (potassium) and NO₃⁻ (nitrate) ions.
- Properties:
- Solubility: Soluble in water.
- Uses: Provides both nitrogen and potassium, essential for plant growth.
3. Compound Fertilisers (NPK Fertilisers)
a. Definition:
- Compound Fertilisers: Mixtures that supply multiple essential nutrients (nitrogen, phosphorus, potassium) in balanced proportions tailored to specific plant needs.
b. NPK Values:
- Understanding NPK Numbers:
- Example: NPK 21:8:11
- 21% Nitrogen (N): Promotes leafy growth.
- 8% Phosphorus (P): Enhances root development.
- 11% Potassium (K): Aids in flower and fruit production.
- Usage: Different crops require different NPK ratios. For instance:
- Fruits (e.g., apples, tomatoes): Higher potassium content.
- Leafy Vegetables (e.g., cabbage): Higher nitrogen content.
- Root Crops (e.g., carrots): Higher phosphorus content.
- Example: NPK 21:8:11
c. Examples of Compound Fertilisers:
- Calcium Ammonium Nitrate (CAN):
- Composition: Contains calcium carbonate (chalk) mixed with ammonium nitrate.
- Formula: Ca(NO₃)₂·CaCO₃
- Benefits: Neutralizes soil acidity caused by ammonium salts.
4. Chemical Reactions in Fertiliser Production
a. Synthesis of Ammonium Nitrate:
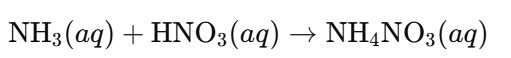
- Process: Ammonia solution reacts with nitric acid to produce ammonium nitrate, which can be crystallised into pellet form for easy application.
b. Solubility and Plant Uptake:
- Ammonium Nitrate and Ammonium Salts:
- Highly Soluble: Ensures that nitrogen is readily available for plant uptake through roots.
- Importance: Soluble nitrogen compounds are essential for effective fertilisation.
c. Neutralizing Soil Acidity:
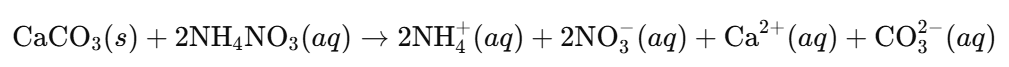
- Explanation: Calcium carbonate (chalk) neutralizes the acidity from ammonium salts, maintaining optimal soil pH for plant growth.
5. Environmental Considerations
a. Pollution from Excessive Fertilisers:
- Eutrophication: Excess nitrates and phosphates run off into water bodies, causing excessive algal growth that depletes oxygen and harms aquatic life.
- Soil Acidification: Overuse of ammonium salts can make soil overly acidic, negatively impacting plant health and soil microorganisms.
- Greenhouse Gas Emissions: Production and application of fertilisers can contribute to greenhouse gas emissions, exacerbating climate change.
b. Sustainable Practices:
- Balanced Application: Using appropriate NPK ratios tailored to specific crops to minimize excess.
- Buffering Soil Acidity: Incorporating calcium carbonate or other neutralizing agents to maintain soil pH.
- Precision Agriculture: Employing technologies to apply fertilisers more efficiently and reduce runoff.
6. Key Terminology
- Fertilisers: Substances added to soil to supply essential nutrients for plant growth.
- Ammonium Salts: Fertilisers containing the ammonium ion (NH₄⁺), such as ammonium nitrate and ammonium sulfate.
- Nitrates: Fertilisers containing the nitrate ion (NO₃⁻), such as potassium nitrate.
- Compound Fertilisers (NPK): Mixtures providing nitrogen (N), phosphorus (P), and potassium (K).
- Ammonium Nitrate (NH₄NO₃): A nitrogenous fertiliser providing both NH₄⁺ and NO₃⁻ ions.
- Calcium Ammonium Nitrate (CAN): A compound fertiliser that neutralizes soil acidity.
- NPK Values: Numbers indicating the percentage composition of nitrogen, phosphorus, and potassium in fertilisers.
- Eutrophication: Over-enrichment of water bodies with nutrients, leading to excessive plant growth and oxygen depletion.
- Soil Acidification: Decrease in soil pH due to excessive use of certain fertilisers, harming plant growth.
- Precision Agriculture: Farming management concept using technology to optimize field-level management regarding crop farming.
Examples:
Question 1:
a. Why do farmers use fertilisers?
Answer:
- Farmers use fertilisers to replenish essential minerals in the soil that are depleted through continuous crop cultivation. These minerals are necessary for plant growth, enabling plants to produce healthy leaves, roots, flowers, and fruits, and thereby increasing crop yields to meet the food demands of the growing population.
b. Why do many fertilisers contain the elements N, P, and K?
Answer:
- Nitrogen (N): Essential for the production of proteins and healthy foliage.
- Phosphorus (P): Important for root development and energy transfer within plants.
- Potassium (K): Vital for flower and fruit production, water regulation, and overall plant health.
- These three elements are the most essential nutrients that plants require in large quantities, making NPK fertilisers effective for promoting balanced and vigorous plant growth.
c. Fertilisers are used by farmers for beneficial effects, but how can excessive or inappropriate use of fertilisers cause pollution (Chapter 17)?
Answer:
- Greenhouse Gas Emissions: Production and application of fertilisers can release greenhouse gases like nitrous oxide, contributing to climate change.
- Eutrophication: Excess nitrates and phosphates from fertilisers can runoff into water bodies, promoting excessive algal blooms that deplete oxygen in the water, harming aquatic life.
- Soil Acidification: Overuse of ammonium salts can lower soil pH, negatively affecting plant health and soil microorganisms.
- Groundwater Contamination: High levels of nitrates can leach into groundwater, making it unsafe for human consumption.
Question 2:
State the names of an acid and alkali that could be used to make the following fertilisers.
a. Ammonium Nitrate (NH₄NO₃):
Answer:
- Acid: Nitric acid (HNO₃)
- Alkali: Ammonia (NH₃) acts as a weak base (alkali)
Reaction:
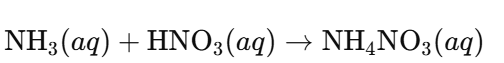
b. Ammonium Phosphate ((NH₄)₃PO₄):
Answer:
- Acid: Phosphoric acid (H₃PO₄)
- Alkali: Ammonia (NH₃) acts as a weak base (alkali)
Reaction:
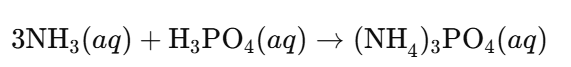
c. Ammonium Sulfate ((NH₄)₂SO₄):
Answer:
- Acid: Sulfuric acid (H₂SO₄)
- Alkali: Ammonia (NH₃) acts as a weak base (alkali)
Reaction:
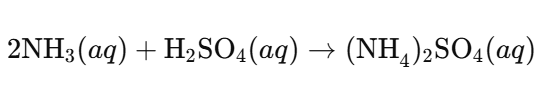
Quizzes:
Quiz 1
Quiz 2