9.01 Reversible Reactions
1. Overview of Reversible Reactions
a. Definition:
- Reversible Reaction: A chemical reaction where the reactants form products, which can subsequently react to form the original reactants. These reactions can proceed in both the forward and reverse directions.
b. Importance:
- Reversible reactions are fundamental in both biological systems and industrial processes.
- Understanding reversible reactions helps explain phenomena like respiration, synthesis of chemicals, and equilibrium states in chemical systems.
2. Examples of Reversible Reactions
a. Biological Example: Hemoglobin and Oxygen
- Process:
- Binding: Oxygen (O₂) binds reversibly to hemoglobin (Hb) in red blood cells within the lungs.
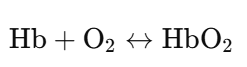
- Release: Oxygen is released from hemoglobin in the tissues where it is needed.
- Significance:
- Essential for transporting oxygen throughout the body.
- The reversibility allows oxygen to be picked up in the lungs and released in the tissues based on changing conditions (e.g., oxygen concentration, pH).
b. Industrial Example: Haber Process
- Reaction:
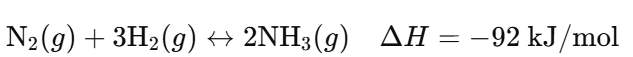
- Forward Reaction: Nitrogen and hydrogen gases combine to form ammonia (NH₃).
- Reverse Reaction: Ammonia decomposes back into nitrogen and hydrogen gases.
- Significance:
- Fritz Haber developed this process to produce ammonia on an industrial scale, essential for fertilizers.
- Control of conditions (pressure, temperature, catalysts) is crucial to maximize ammonia production.
c. Reversible Hydration of Salts
- Example: Hydrated Copper(II) Sulfate
- Dehydration (Heating):
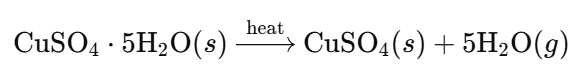
- Observation: Blue crystals (hydrated) turn white (anhydrous) upon heating.
- Hydration (Adding Water):
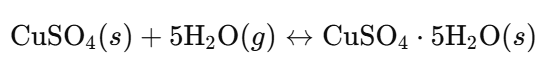
- Observation: White powder turns back to blue crystals when water is added.
- Other Examples:
- Cobalt(II) Chloride: Pink hydrated form becomes blue when dehydrated.
- Iron(II) Sulfate: Similar reversible hydration-dehydration behavior.
3. Chemical Equilibrium
a. Definition:
- Chemical Equilibrium: A state in a reversible reaction where the concentrations of reactants and products remain constant because the forward and reverse reactions occur at the same rate.
b. Dynamic Equilibrium:
- Nature: Even though the concentrations remain constant, both forward and reverse reactions are continuously occurring.
- Analogy: A person running up an escalator moving down at the same speed appears stationary.
c. Closed Systems:
- Definition: Systems where no reactants or products can escape, allowing equilibrium to be established.
- Open Systems: Reactions may reach equilibrium without a true balance of reactants and products.
d. Position of Equilibrium:
- To the Right: More products are formed.
- To the Left: More reactants remain.
- Depends on Conditions: Temperature, pressure, concentration, and catalysts can shift the equilibrium position.
4. Factors Affecting Equilibrium
a. Temperature:
- Exothermic Reactions (Release Heat):
- Increase Temperature: Shifts equilibrium to the left (favoring reactants).
- Decrease Temperature: Shifts equilibrium to the right (favoring products).
- Endothermic Reactions (Absorb Heat):
- Increase Temperature: Shifts equilibrium to the right (favoring products).
- Decrease Temperature: Shifts equilibrium to the left (favoring reactants).
b. Pressure:
- Applicable to Gaseous Reactions:
- Increase Pressure: Shifts equilibrium toward the side with fewer gas molecules.
- Decrease Pressure: Shifts equilibrium toward the side with more gas molecules.
c. Concentration:
- Increase in Reactant/Product:
- Reactant: Shifts equilibrium to the right.
- Product: Shifts equilibrium to the left.
- Decrease in Reactant/Product:
- Reactant: Shifts equilibrium to the left.
- Product: Shifts equilibrium to the right.
d. Catalysts:
- Effect: Speed up both forward and reverse reactions equally.
- Equilibrium Position: Remains unchanged.
- Role: Helps reach equilibrium faster.
5. Le Chatelier’s Principle
a. Definition:
- Le Chatelier’s Principle: If a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium moves to counteract the change.
b. Application:
- Temperature Changes: As described above.
- Pressure Changes: As described above.
- Concentration Changes: As described above.
- Catalysts: Do not affect the position of equilibrium.
6. Key Terminology
- Reversible Reaction: A reaction that can proceed in both forward and reverse directions.
- Chemical Equilibrium: A state where the forward and reverse reactions occur at the same rate.
- Dynamic Equilibrium: Continuous forward and reverse reactions with no net change in concentrations.
- Closed System: A system where no substances can enter or leave during the reaction.
- Le Chatelier’s Principle: Predicts how changes in conditions affect equilibrium.
- Exothermic Reaction: Releases heat.
- Endothermic Reaction: Absorbs heat.
- Cation: A positively charged ion.
- Anion: A negatively charged ion.
- Hydrated Salt: A salt containing water molecules within its crystal structure.
- Anhydrous: Without water.
- Catalyst: A substance that speeds up a reaction without being consumed.
Examples:
1.1 What colour change do we see when water is added to anhydrous copper(II) sulfate powder?
Answer:
- Colour Change: White anhydrous copper(II) sulfate turns blue when water is added, forming hydrated copper(II) sulfate.
1.2 What can the colour change seen in part a be used as a test for?
Answer:
- Test for Presence of Water: The colour change from white to blue indicates the presence of water.
1.2 Write a balanced symbol equation for the reaction in part a.
Answer:
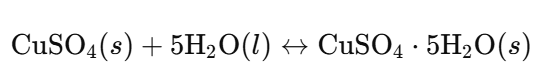
2. Which test would you carry out to show that a colourless liquid was pure water?
Answer:
- Boiling Point Test: Measure the boiling point to confirm it is exactly 100°C.
- Additional Test: Use cobalt(II) chloride paper, which remains blue only in the absence of water. If the liquid turns the paper pink, water is present, but further tests are needed to confirm purity.
3. How do we know that the reaction to form pink hydrated cobalt(II) chloride from blue anhydrous cobalt(II) chloride is exothermic?
Answer:
- Observation: The colour change from blue to pink releases heat, indicating that the reverse reaction (hydration) is exothermic.
4. Which of the following elements exists as a giant covalent structure?
- A. Carbon
- B. Iodine
- C. Helium
- D. Oxygen
Answer:
- A. Carbon
- Explanation: Carbon exists as diamond and graphite, both of which are giant covalent structures.
5. Metals such as copper are bonded together by the attraction between metal ions and a “sea” of delocalised electrons surrounding them. Which of the properties of metals (A–D) is not explained by this type of bonding?
A. Electrical conductivity
B. Malleability
C. Melting point
D. Reaction with acids
Answer:
Explanation: Reaction with acids involves the chemical reactivity of metals, which is influenced by factors beyond just metallic bonding, such as the metal’s position in the reactivity series.
D. Reaction with acid
Quizzes:
Quiz 1
Quiz 2