19.21 Chapter Summary
BioCast:
1. Definition of Recombinant DNA
- Recombinant DNA refers to DNA molecules formed by combining genetic material from different sources. This process involves inserting a gene from one organism into the DNA of another, creating a new genetic combination that does not occur naturally. Recombinant DNA technology is fundamental in genetic engineering, allowing scientists to introduce desired traits into organisms.
Key Points:
- Recombinant DNA is artificially created by combining DNA from different sources.
- Essential for genetic engineering and biotechnology applications.
- Enables the expression of specific genes in host organisms.
2. Genetic Engineering: Deliberate Manipulation of Genetic Material
- Genetic Engineering is the intentional modification of an organism’s genetic makeup to alter specific characteristics. This process often involves transferring a gene from one organism to another to ensure that the gene is expressed and imparts the desired trait.
Key Points:
- Deliberate Manipulation: Purposeful changes made to an organism’s DNA.
- Modification of Characteristics: Traits such as disease resistance, growth rate, or biochemical production can be targeted.
- Gene Transfer: Introducing a gene into an organism so that it is expressed and functional.
Example:
- Inserting the insulin gene from humans into bacteria to produce insulin for diabetic patients.
3. Sources of Genes for Transfer
- Genes intended for transfer into an organism can be obtained through various methods:
a. Extracted from the DNA of a Donor Organism:
- Process: Isolate DNA from the donor organism using cell lysis and purification techniques.
- Enzyme Use: Restriction endonucleases may be used to cut the DNA at specific sites.
b. Synthesized from the mRNA of a Donor Organism:
- Process: Extract mRNA from the donor, then use reverse transcriptase to synthesize complementary DNA (cDNA).
- Application: Useful when only the expressed genes (mRNA) are needed.
c. Synthesized Chemically from Nucleotides:
- Process: Chemically assemble the desired DNA sequence using nucleotides.
- Advantages: Allows for precise control over the DNA sequence, including the introduction of mutations or specific modifications.
Key Points:
- Multiple methods to obtain genes ensure flexibility in genetic engineering.
- Choice of method depends on the source material and desired outcome.
4. Roles of Key Enzymes and Vectors in Gene Transfer
a. Restriction Endonucleases:
- Function: Cut DNA at specific recognition sites, creating fragments with sticky or blunt ends.
- Importance: Enables precise insertion of genes into vectors by creating compatible ends.
b. DNA Ligase:
- Function: Joins DNA fragments by forming phosphodiester bonds.
- Importance: Seals the recombinant DNA by linking the inserted gene to the vector DNA.
c. Plasmids:
- Definition: Circular, double-stranded DNA molecules found in bacteria.
- Function: Serve as vectors to carry and replicate the inserted gene within host cells.
- Features: Often contain antibiotic resistance genes for selection.
d. DNA Polymerase:
- Function: Synthesizes new DNA strands during DNA replication.
- Role in Gene Transfer: Replicates the recombinant DNA within the host organism.
e. Reverse Transcriptase:
- Function: Synthesizes DNA from an RNA template.
- Importance: Essential for creating cDNA from mRNA, enabling the transfer of expressed genes.
Key Points:
- Restriction Endonucleases and DNA Ligase are critical for cutting and joining DNA fragments.
- Plasmids act as carriers for recombinant DNA in host organisms.
- DNA Polymerase ensures replication of the recombinant DNA, while Reverse Transcriptase allows the use of RNA templates.
5. The Role of Promoters in Gene Transfer
- A promoter is a DNA sequence that initiates transcription of a gene. For a transferred gene to be expressed in the host organism, a promoter must be present to ensure that the host’s RNA polymerase can recognize and transcribe the gene.
Key Points:
- Promoter Function: Controls the expression of the inserted gene by signaling where transcription should start.
- Necessity: Without a promoter, the host organism may not express the transferred gene, rendering the genetic modification ineffective.
- Types: Promoters can be constitutive (always active) or inducible (activated under specific conditions).
Example:
- Using a bacterial promoter to drive the expression of a human gene in bacterial cells.
6. Confirming Gene Expression with Marker Genes
- Marker Genes are used to verify that the desired gene has been successfully transferred and is being expressed in the host organism. One common method involves using genes that code for fluorescent products.
Key Points:
- Fluorescent Marker Genes: Encode proteins that emit fluorescence, making it easy to identify transformed cells.
- Verification: Presence of fluorescence indicates successful gene transfer and expression.
- Examples: Green Fluorescent Protein (GFP) is widely used as a marker.
Procedure:
- Insert both the desired gene and the marker gene into the vector.
- Transform host cells with the recombinant DNA.
- Expose cells to UV light or specific wavelengths to detect fluorescence.
- Identify and select fluorescent cells as successfully transformed.
7. Gene Editing as a Form of Genetic Engineering
- Gene Editing involves making precise changes to an organism’s genome, including the insertion, deletion, or replacement of DNA at specific locations. This technique allows for targeted modifications without introducing foreign DNA.
Key Points:
- Precision: Enables specific alterations at designated genomic sites.
- Techniques: Includes CRISPR-Cas9, TALENs, and ZFNs.
- Applications: Correcting genetic disorders, improving crop traits, and studying gene function.
Example:
- Using CRISPR-Cas9 to delete a faulty gene responsible for a genetic disease, thereby restoring normal function.
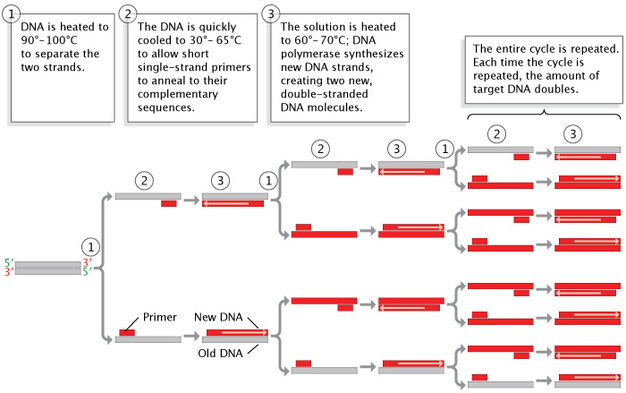
8. Polymerase Chain Reaction (PCR)
- Polymerase Chain Reaction (PCR) is a technique used to amplify specific DNA sequences, enabling the cloning and study of genes.
Steps Involved in PCR:
- Denaturation: Heating the DNA to separate the two strands.
- Annealing: Cooling the mixture to allow primers to bind to the target sequences.
- Extension: DNA polymerase extends the primers, synthesizing new DNA strands.
Role of Taq Polymerase:
- Definition: A heat-stable DNA polymerase derived from the bacterium Thermus aquaticus.
- Function: Remains active at high temperatures used during the denaturation step, allowing multiple cycles of PCR without enzyme degradation.
- Importance: Facilitates the rapid and efficient amplification of DNA.
Key Points:
- PCR can exponentially amplify specific DNA regions from minute samples.
- Applications: Genetic cloning, forensic analysis, medical diagnostics, and research.
Diagram:
[Diagram of PCR Cycle]
- Denaturation (94-98°C)
- Annealing (50-65°C)
- Extension (72°C)
Repeat for 25-35 cycles
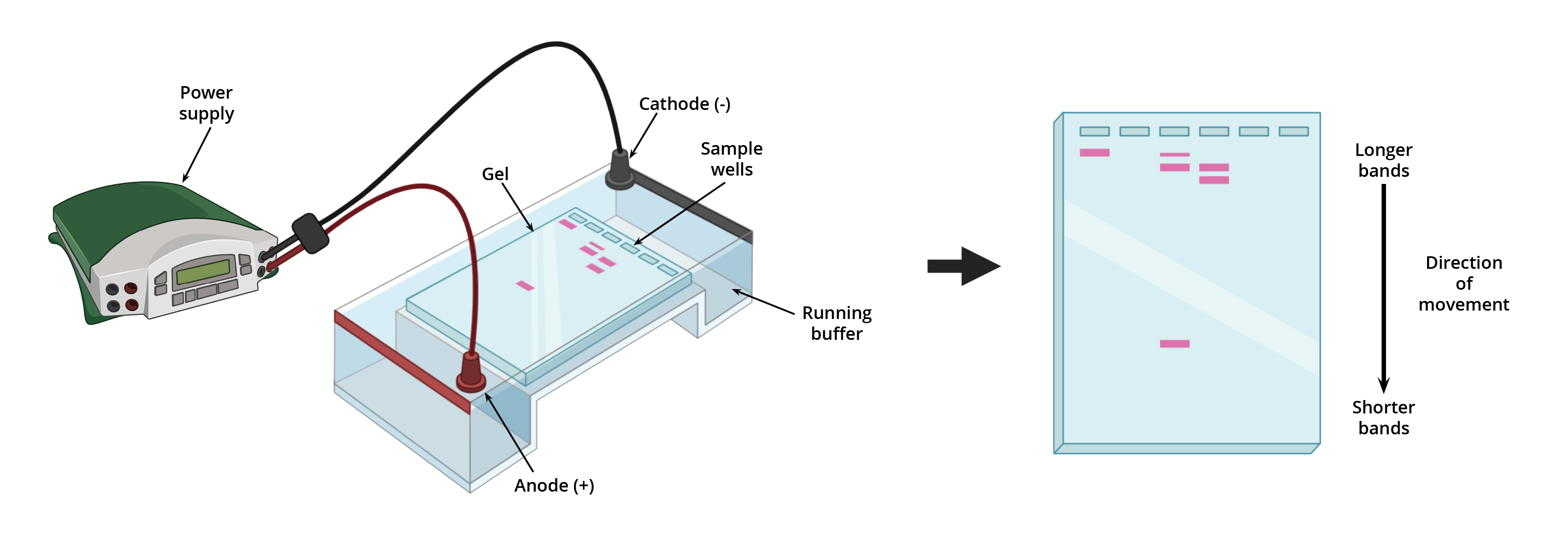
9. Gel Electrophoresis for DNA Fragment Separation
- Gel Electrophoresis is a technique used to separate DNA fragments based on their size by applying an electric field to a gel matrix.
Procedure:
- Preparation: Agarose gel is prepared with wells for sample loading.
- Loading Samples: DNA samples mixed with a loading dye are placed into the wells.
- Applying Electric Field: DNA fragments migrate towards the positive electrode (since DNA is negatively charged).
- Separation: Smaller fragments move faster and travel farther through the gel matrix, while larger fragments move slower.
Interpretation:
- Bands: Each band represents DNA fragments of a specific size.
- Comparison: DNA fragments can be compared against a DNA ladder (size standard) to determine their lengths.
Key Points:
- Resolution: Agarose concentration affects the resolution of DNA fragment sizes.
- Visualization: DNA is typically stained with ethidium bromide or other dyes and visualized under UV light.
- Applications: Verifying PCR products, analyzing restriction digests, and checking plasmid integrity.
Diagram:
[Agarose Gel with DNA Bands]
10. Microarrays in Genome Analysis and Gene Expression Studies
- Microarrays are tools that allow the simultaneous analysis of thousands of genes, providing comprehensive data on genome structure and gene expression patterns.
Uses in Genome Analysis:
- Gene Identification: Determine which genes are present in a genome.
- Genetic Variation: Detect single nucleotide polymorphisms (SNPs) and other genetic variations.
Uses in Gene Expression:
- mRNA Detection: Measure the expression levels of multiple genes by detecting their mRNA transcripts.
- Comparative Studies: Compare gene expression profiles under different conditions (e.g., healthy vs. diseased tissues).
Procedure:
- Sample Preparation: Extract mRNA from the cells or tissues of interest.
- Labeling: Convert mRNA to cDNA and label with fluorescent dyes.
- Hybridization: Apply labeled cDNA to the microarray, where it binds to complementary DNA probes on the array.
- Scanning and Analysis: Use a scanner to detect fluorescence intensity, indicating gene expression levels.
Key Points:
- High-Throughput: Enables the analysis of thousands of genes simultaneously.
- Data Interpretation: Requires bioinformatics tools to handle large datasets and identify significant expression changes.
- Applications: Cancer research, developmental biology, and personalized medicine.
11. Benefits of Genetic and Protein Databases
- Genetic and Protein Databases store vast amounts of information regarding nucleotide sequences of genes, genomes, amino acid sequences of proteins, and protein structures. These databases are invaluable resources for researchers and clinicians.
Benefits:
- Accessibility: Provide easy access to genetic and protein information from diverse organisms.
- Research Facilitation: Support comparative genomics, evolutionary studies, and functional genomics.
- Data Integration: Combine sequence data with functional annotations, facilitating a deeper understanding of gene and protein functions.
- Drug Development: Aid in identifying potential drug targets by analyzing protein structures and functions.
- Educational Resource: Serve as a learning tool for students and educators in genetics and molecular biology.
Examples of Databases:
- GenBank: A comprehensive database of publicly available nucleotide sequences.
- Protein Data Bank (PDB): Contains 3D structural data of proteins and nucleic acids.
- UniProt: A detailed resource for protein sequence and functional information.
Key Points:
- Efficiency: Save time and resources by providing readily available genetic and protein information.
- Collaboration: Enable global collaboration by sharing data across research institutions.
- Advancement of Science: Accelerate discoveries by providing a foundation of existing knowledge.
12. Advantages of Using Recombinant Human Proteins to Treat Disease
Recombinant DNA Technology Overview:
- Definition: The process of combining DNA from different sources to create new genetic combinations.
- Method: Involves inserting human genes into bacterial plasmids, which are then introduced into bacterial cells (e.g., E. coli) to produce the desired protein.
Advantages:
- Purity and Safety:
- Produced in controlled environments, minimizing contamination with pathogens.
- Higher purity compared to proteins extracted from human or animal tissues.
- Consistency and Reliability:
- Standardized production ensures consistent dosage and efficacy.
- Cost-Effectiveness:
- Large-scale production reduces costs compared to extraction from natural sources.
- Reduced Immune Response:
- Human proteins are less likely to be recognized as foreign by the patient’s immune system, decreasing the risk of adverse reactions.
- Ethical Considerations:
- Avoids ethical issues related to sourcing proteins from human or animal tissues.
Examples:
- Insulin:
- Use: Treatment of diabetes mellitus.
- Production: Human insulin gene inserted into E. coli or yeast cells to produce insulin identical to that produced by the human pancreas.
- Advantages:
- Eliminates the need for insulin extraction from animal pancreases, reducing the risk of allergic reactions.
- Provides a reliable and continuous supply to meet global demand.
- Factor VIII:
- Use: Treatment of Hemophilia A.
- Production: Recombinant Factor VIII produced in genetically modified mammalian cells.
- Advantages:
- Provides a safe and effective treatment option without the risk of blood-borne infections associated with plasma-derived Factor VIII.
- Adenosine Deaminase (ADA):
- Use: Treatment of Severe Combined Immunodeficiency (SCID).
- Production: Recombinant ADA produced using bacterial or mammalian cells.
- Advantages:
- Restores immune function in patients with ADA deficiency, a genetic disorder affecting the immune system.
13. Advantages of Genetic Screening
Genetic Screening Overview:
- Definition: Testing individuals or populations to identify genetic disorders or predispositions.
- Purpose: Early diagnosis, prevention, and management of genetic diseases.
Advantages:
- Early Detection:
- Identifies carriers and affected individuals before symptoms appear, allowing for timely intervention.
- Informed Decision-Making:
- Enables individuals to make informed choices about family planning and lifestyle.
- Disease Prevention:
- Reduces incidence of genetic disorders through carrier screening and prenatal diagnosis.
- Personalized Medicine:
- Tailors treatments based on genetic profiles, improving efficacy and reducing side effects.
- Public Health Benefits:
- Enhances overall health outcomes and reduces healthcare costs by preventing or mitigating genetic diseases.
Examples:
- Breast Cancer (BRCA1 and BRCA2 Genes):
- Genes Involved: BRCA1 and BRCA2 mutations significantly increase the risk of breast and ovarian cancers.
- Screening Benefits:
- Identifies individuals at high risk, allowing for proactive monitoring, preventive surgeries, or lifestyle changes to reduce cancer risk.
- Huntington’s Disease:
- Gene Involved: Mutation in the HTT gene leads to the progressive neurodegenerative disorder.
- Screening Benefits:
- Predictive testing for at-risk individuals can inform life planning and family decisions, despite the absence of a cure.
- Cystic Fibrosis:
- Gene Involved: Mutations in the CFTR gene cause cystic fibrosis, affecting the respiratory and digestive systems.
- Screening Benefits:
- Carrier screening for prospective parents can inform reproductive choices, such as in vitro fertilization with genetic testing to avoid passing the disorder to offspring.
14. Treating Genetic Diseases with Gene Therapy
Gene Therapy Overview:
- Definition: A technique that modifies a person’s genes to treat or prevent disease by correcting defective genes or introducing new ones.
- Approaches:
- Somatic Gene Therapy: Targets non-reproductive cells; changes are not passed to offspring.
- Germline Gene Therapy: Targets reproductive cells; changes are heritable (currently not widely practiced due to ethical concerns).
Treatment Advantages:
- Potential Cure: Offers the possibility of curing genetic disorders rather than just managing symptoms.
- Targeted Therapy: Directly addresses the underlying genetic cause of the disease.
- Long-Lasting Effects: Can provide permanent solutions with a single treatment.
Examples:
- Severe Combined Immunodeficiency (SCID):
- Condition: A genetic disorder characterized by a severely compromised immune system.
- Gene Therapy Approach:
- Introduction of a functional ADA gene into the patient’s hematopoietic stem cells.
- Outcome: Restoration of immune function by enabling the production of functional ADA enzyme, essential for immune cell development.
- Inherited Eye Diseases:
- Condition: Conditions like Leber’s Congenital Amaurosis (LCA) cause severe vision loss or blindness.
- Gene Therapy Approach:
- Delivery of a normal copy of the RPE65 gene to retinal cells using viral vectors.
- Outcome: Restoration of vision by enabling retinal cells to produce the necessary protein for photoreceptor function.
15. Social and Ethical Considerations of Genetic Screening and Gene Therapy
Social Considerations:
- Accessibility and Equity:
- Ensuring that genetic technologies are accessible to all segments of society, regardless of socioeconomic status.
- Discrimination and Stigmatization:
- Risk of genetic information being used to discriminate in employment, insurance, or social settings.
- Psychological Impact:
- Individuals may experience anxiety, stress, or altered self-perception upon learning their genetic risks.
Ethical Considerations:
- Consent and Autonomy:
- Informed Consent: Ensuring individuals fully understand the implications of genetic testing and gene therapy before consenting.
- Autonomy: Respecting individuals’ rights to make decisions about their genetic information and treatments without coercion.
- Privacy and Confidentiality:
- Protecting individuals’ genetic information from unauthorized access and ensuring confidentiality to prevent misuse.
- Genetic Determinism:
- Avoiding the misconception that genes solely determine health and behavior, acknowledging the role of environment and lifestyle.
- Equity in Access to Treatments:
- Addressing disparities in access to genetic therapies to prevent widening health inequalities.
- Ethical Boundaries of Gene Editing:
- Somatic vs. Germline Editing: Somatic gene therapy is generally accepted, while germline editing raises significant ethical concerns due to its heritable nature.
- Enhancement vs. Therapy: Distinguishing between therapeutic uses (treating diseases) and enhancement (improving non-disease traits), which raises ethical debates about human enhancement.
- Consent in Vulnerable Populations:
- Ensuring that minors or individuals with impaired decision-making capacities receive appropriate protection and consent processes.
- Long-Term Implications:
- Considering the long-term effects and potential unintended consequences of genetic interventions on individuals and future generations.
Discussion Points:
- Balancing the benefits of genetic technologies with the potential risks and ethical dilemmas.
- Developing robust legal and regulatory frameworks to govern the use of genetic screening and gene therapy.
- Promoting public awareness and education to foster informed decision-making and ethical use of genetic technologies.
16. Genetic Engineering and Global Food Demand
- Genetic engineering involves the direct manipulation of an organism’s genome using biotechnology. In agriculture, it aims to improve crop and animal characteristics to meet the growing global food demand. This section explores how genetic engineering enhances quality and productivity, focusing on specific examples: GM salmon, herbicide-resistant soybean, and insect-resistant cotton.
1.1. Improving Quality and Productivity Through Genetic Engineering
a. Enhanced Crop Yields
- Increased Productivity: Genetic modifications can lead to higher yields per hectare by making crops more efficient in nutrient uptake, growth rate, and resistance to diseases and pests.
- Stress Resistance: GM crops can be engineered to withstand abiotic stresses such as drought, salinity, and extreme temperatures, ensuring stable production even under adverse conditions.
b. Improved Nutritional Quality
- Biofortification: Genetic engineering can enhance the nutritional content of crops, such as increasing vitamin, mineral, and protein levels to combat malnutrition.
- Quality Traits: Traits like improved taste, longer shelf-life, and better appearance can be achieved, making crops more appealing to consumers and reducing post-harvest losses.
c. Enhanced Farmed Animal Productivity
- Growth Rate: Genetically modified animals can exhibit faster growth rates, leading to increased meat, milk, or egg production.
- Disease Resistance: Engineering animals to resist specific diseases reduces mortality rates and the need for antibiotics, promoting healthier livestock populations.
1.2. Case Studies
a. Genetically Modified Salmon
- Development: The AquAdvantage salmon is the first genetically modified animal approved for human consumption. It contains a growth hormone-regulating gene from Chinook salmon and a promoter from ocean pout, enabling it to grow year-round instead of only during spring and summer.
- Benefits:
- Faster Growth: The GM salmon can reach market size in approximately half the time of non-GM counterparts, reducing production costs.
- Efficiency: More efficient use of resources due to faster growth rates contributes to increased overall productivity.
- Considerations:
- Regulatory Approval: GM salmon has undergone rigorous safety assessments by regulatory bodies like the FDA.
- Environmental Impact: Containment measures are essential to prevent potential interbreeding with wild salmon populations.
b. Herbicide-Resistant Soybean
- Development: Herbicide-resistant soybean varieties, such as those resistant to glyphosate (e.g., Roundup Ready), have been engineered to survive applications of specific herbicides.
- Benefits:
- Weed Control: Farmers can effectively control weeds without damaging the soybean crop, leading to higher yields.
- Simplified Farming Practices: Reduced need for mechanical weed control decreases labor and fuel costs.
- Considerations:
- Herbicide Use: Overreliance on a single herbicide can lead to the evolution of herbicide-resistant weed species, necessitating integrated weed management strategies.
- Environmental Impact: Glyphosate’s effects on non-target organisms and soil health require ongoing monitoring.
c. Insect-Resistant Cotton
- Development: Bt cotton incorporates genes from the bacterium Bacillus thuringiensis (Bt), which produce proteins toxic to specific insect pests, such as the bollworm.
- Benefits:
- Pest Control: Reduced damage from insect pests leads to higher cotton yields and quality.
- Reduced Pesticide Use: Decreased need for chemical insecticides lowers production costs and minimizes environmental contamination.
- Considerations:
- Resistance Management: Continuous monitoring and management strategies are essential to prevent pests from developing resistance to Bt toxins.
- Non-target Effects: Potential impacts on beneficial insects and biodiversity must be assessed and mitigated.
17. Ethical and Social Implications of GMOs in Food Production
While GMOs offer significant benefits in agriculture, their use raises various ethical and social concerns. This section delves into the primary issues associated with GMOs in food production.
2.1. Ethical Considerations
a. Food Safety and Health Concerns
- Safety Assessment: Rigorous testing is required to ensure that GM foods are safe for human consumption. However, public skepticism remains due to perceived unknown long-term effects.
- Allergenicity: Introduction of new genes may inadvertently create allergens. Ensuring thorough testing is crucial to prevent adverse health effects.
b. Environmental Ethics
- Biodiversity: GM crops can potentially crossbreed with wild relatives, leading to reduced genetic diversity and unforeseen ecological consequences.
- Ecosystem Balance: Traits like pest resistance may disrupt natural predator-prey relationships and affect non-target species.
c. Animal Welfare
- GM Animals: Enhancing growth rates or disease resistance in animals raises questions about their well-being and the ethical implications of modifying sentient beings for human benefit.
2.2. Social Implications
a. Economic Impact
- Farmers’ Dependence: Patent protections on GM seeds by large corporations can make farmers dependent on purchasing seeds each season, increasing their costs and reducing autonomy.
- Market Access: Developing countries may face barriers in accessing GM technologies, potentially widening economic disparities.
b. Labeling and Consumer Choice
- Transparency: Consumers advocate for clear labeling of GM foods to make informed choices. Mandatory labeling debates highlight the balance between consumer rights and industry interests.
- Public Perception: Misinformation and lack of understanding about genetic engineering can lead to resistance against GMOs, affecting market acceptance.
c. Intellectual Property and Biotechnology Control
- Corporate Control: A few multinational companies dominate the GMO market, raising concerns about monopolistic practices and fair distribution of benefits.
- Access to Technology: Ensuring equitable access to genetic engineering technologies is essential for global food security without exacerbating inequalities.
2.3. Societal and Cultural Factors
a. Cultural Acceptance
- Traditional Practices: In some cultures, genetically modifying organisms may conflict with traditional farming practices and beliefs about natural food.
- Cultural Heritage: Preserving indigenous crops and biodiversity can be threatened by the widespread adoption of GM crops.
b. Global Food Security
- Potential Benefits: GMOs can contribute significantly to food security by increasing yields and enhancing nutritional content, particularly in regions facing food shortages.
- Distribution Challenges: Ensuring that the benefits of GMOs reach marginalized and resource-poor populations is critical for addressing global hunger.
2.4. Regulatory and Governance Issues
a. International Regulations
- Diverse Policies: Countries have varying regulations regarding GMO approval, cultivation, and import/export, affecting global trade and cooperation.
- Harmonization Efforts: International bodies like the Codex Alimentarius work towards standardizing safety assessments and labeling requirements.
b. Public Participation
- Stakeholder Engagement: Involving farmers, consumers, scientists, and policymakers in decision-making processes ensures that diverse perspectives are considered.
- Transparency: Open communication about the risks and benefits of GMOs fosters trust and informed public discourse.