11.01 Acids
1.1 What is an Acid?
Definition:
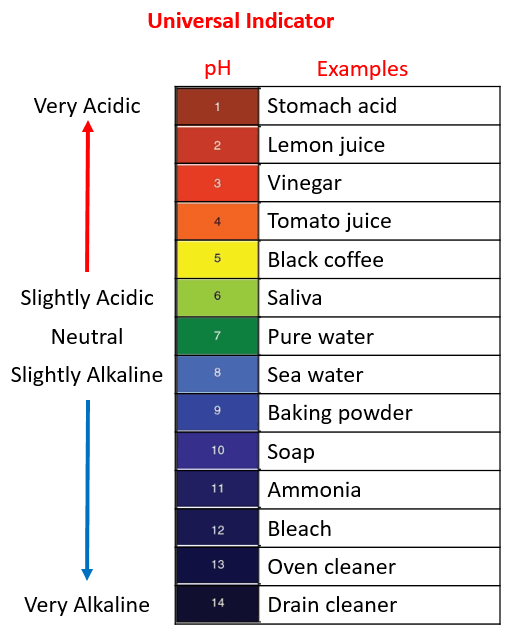
- Acid: A substance that dissolves in water to produce hydrogen ions (H⁺). An acid solution turns blue litmus paper red and has a pH below 7.
- Proton Donor: Acids act as proton donors in chemical reactions, meaning they can donate H⁺ ions to other substances.

Examples:
1. Hydrochloric Acid (HCl)
- HCl is a strong acid that dissociates completely in water: HCl → H⁺ + Cl⁻
- HCl donates a proton (H⁺) to water, leaving behind the chloride ion (Cl⁻).
2. Sulfuric Acid (H₂SO₄)
- H₂SO₄ is a strong acid that donates one proton in the first dissociation step: H₂SO₄→H⁺+HSO₄⁻
- The resulting HSO₄⁻ (bisulfate ion) can act as a weak acid and donate a second proton:
3. Acetic Acid (CH₃COOH)
- CH₃COOH is a weak acid that donates a proton to water: CH₃COOH + H₂O ↔ CH₃COO⁻ + H₃O⁺
- Acetic acid donates a proton (H⁺) to water, forming the acetate ion (CH₃COO⁻) and hydronium ion (H₃O⁺).
4. Carbonic Acid (H₂CO₃)
- H₂CO₃ is a weak acid that donates a proton in the following steps: H₂CO₃ ↔ H⁺ + HCO₃⁻
- The bicarbonate ion (HCO₃⁻) can also act as an acid and donate another proton:
5. Ammonium Ion (NH₄⁺)
- Here, ammonium donates a proton to form ammonia.
- While not a common “acid,” NH₄⁺ demonstrates proton donation: NH₄⁺↔H⁺+NH₃
Characteristics of Acids:
- Sour Taste: Many acids taste sour (e.g., vinegar, lemon juice). Caution: Taste is not a reliable test for acidity as some acids are dangerous and can cause harm.
- Corrosive Nature: Acids can be corrosive, meaning they can “eat away” at materials like metals, skin, and clothing.
Types of Acids:
- Organic Acids
- Mineral (Inorganic) Acids
2. Types of Acids
2.1 Organic Acids
Definition:
- Organic Acids: Acids that contain carbon and are typically found in plant and animal materials. They are generally weak and dilute.
Common Organic Acids and Examples:
| Acid | Chemical Formula | Common Source/Use | Properties |
|---|---|---|---|
| Methanoic Acid | HCOOH | Formic acid in ant and nettle stings | Weak acid, used in kettle descalers |
| Ethanoic Acid | CH₃COOH | Vinegar | Weak acid, used in vinegar |
| Lactic Acid | C₃H₆O₃ | Sour milk | Weak acid |
| Citric Acid | C₆H₈O₇ | Citrus fruits (lemons, oranges) | Weak acid, found in citrus juices |
| Carbonic Acid | H₂CO₃ | Soft fizzy drinks | Weak acid, formed from CO₂ in water |
Key Points:
- Weak Acids: Do not completely dissociate in water.
- Dilute Solutions: Have lower concentrations of hydrogen ions (H⁺).
2.2 Mineral Acids
Definition:
- Mineral Acids: Also known as inorganic acids, these acids do not contain carbon. They are typically strong, concentrated, and highly corrosive.
Common Mineral Acids and Examples:
| Acid | Chemical Formula | Common Source/Use | Properties |
|---|---|---|---|
| Hydrochloric Acid | HCl | Stomach acid, used in cleaning metal surfaces | Strong acid, highly corrosive |
| Nitric Acid | HNO₃ | Manufacture of fertilizers, explosives | Strong acid, highly corrosive |
| Sulfuric Acid | H₂SO₄ | Car batteries, manufacturing fertilizers | Strong acid, highly corrosive |
| Phosphoric Acid | H₃PO₄ | Used in anti-rust paint, making detergents | Strong acid, used in various industrial processes |
Key Points:
- Strong Acids: Completely dissociate in water, releasing all their hydrogen ions.
- Corrosive Properties: Can cause severe burns and damage materials, including metals and skin.
3. Properties of Acids
3.1 Common Properties
| Property | Description |
|---|---|
| Taste | Sour (e.g., vinegar, lemon juice) |
| pH Level | Less than 7 |
| Litmus Test | Turns blue litmus paper red |
| Proton Donor | Donates H⁺ ions in chemical reactions |
| Corrosiveness | Can damage materials like metals, skin, and clothing |
| Reactivity with Metals | React with certain metals to produce hydrogen gas and salts |
| Reaction with Bases | Neutralization reaction to form water and salts |
3.2 Safety Precautions
- Avoid Direct Contact: Use gloves and eye protection when handling strong acids.
- Proper Ventilation: Work in well-ventilated areas or under a fume hood to avoid inhaling fumes.
- Avoid Ingestion: Do not taste acids as they can be dangerous or deadly.
- Storage: Store acids in appropriate containers, away from incompatible substances.
4. Acids in Everyday Life
4.1 Common Sources of Acids
| Source | Type of Acid | Use/Occurrence |
|---|---|---|
| Vinegar | Ethanoic Acid (CH₃COOH) | Culinary uses, cleaning agent |
| Lemon Juice | Citric Acid (C₆H₈O₇) | Culinary uses, flavoring agent |
| Soft Drinks | Carbonic Acid (H₂CO₃) | Fizz and carbonation |
| Sour Milk | Lactic Acid (C₃H₆O₃) | Fermentation and dairy products |
| Ant and Nettle Stings | Methanoic Acid (HCOOH) | Defensive mechanisms of insects and plants |
| Stomach Acid | Hydrochloric Acid (HCl) | Digestive processes in humans and animals |
| Car Batteries | Sulfuric Acid (H₂SO₄) | Electrolyte in lead-acid batteries |
| Cleaning Agents | Various Mineral Acids | Removal of rust, scale, and other residues |
4.2 Applications of Acids
- Industrial Manufacturing: Production of fertilizers, explosives, and detergents.
- Household Cleaning: Use of diluted mineral acids to clean metal surfaces and remove stains.
- Food Industry: Use of organic acids as preservatives and flavor enhancers.
- Medical Field: Stomach acid (hydrochloric acid) aids in digestion.
5. Identifying Acids and Their Strength
5.1 Weak vs. Strong Acids
| Characteristic | Weak Acids | Strong Acids |
|---|---|---|
| Degree of Dissociation | Partially dissociate in water | Completely dissociate in water |
| Conductivity | Lower electrical conductivity due to fewer ions | High electrical conductivity due to more ions |
| Reaction Rate | Slower reactions with metals and bases | Faster and more vigorous reactions |
| Examples | Ethanoic Acid, Citric Acid, Lactic Acid | Hydrochloric Acid, Sulfuric Acid, Nitric Acid |
Key Points:
- Weak Acids: Do not release all their hydrogen ions in solution, resulting in a less acidic solution.
- Strong Acids: Release all their hydrogen ions in solution, making the solution highly acidic.
Examples:
Question A: What is the oxidation number of the element underlined in the following elements, compounds, and ions?
a. Al(s)
- Element: Aluminium in its elemental form.
- Oxidation Number: 0
b. Cl₂(g)
- Element: Chlorine in its elemental form.
- Oxidation Number: 0
c. I⁻(aq)
- Ion: Iodide ion.
- Oxidation Number: -1
d. Cl⁻(aq)
- Ion: Chloride ion.
- Oxidation Number: -1
e. Cr₂O₇²⁻(aq)
- Ion: Dichromate ion.
- Oxidation Number of Cr:
- Let the oxidation number of Cr = x
- Equation: 2x + 7(−2)= −2
- 2x−14=−2
- 2x=+12
- x=+6
- Oxidation Number of Cr: +6
Question B: Which is the oxidising agent in each of the following reactions?
a. Mg(s)+ZnSO4(aq)→MgSO4(aq)+Zn(s)
Analysis:
- Assign Oxidation Numbers:
- Mg: 0 → +2 (oxidation)
- Zn: +2 → 0 (reduction)
- Oxidising Agent: Zn²⁺ (from ZnSO₄) as it gains electrons (is reduced).
b. Br2(aq)+2KI(aq)→2KBr(aq)+I2(aq)
Analysis:
- Assign Oxidation Numbers:
- Br₂: 0 → -1 (reduction)
- I⁻: -1 → 0 (oxidation)
- Oxidising Agent: Br₂ as it gains electrons (is reduced).
c. 5Fe2+(aq)+MnO4−(aq)+8H+(aq)→5Fe3+(aq)+Mn2+(aq)+4H2O(l)
Analysis:
- Assign Oxidation Numbers:
- Fe²⁺: +2 → Fe³⁺: +3 (oxidation)
- MnO₄⁻: +7 → Mn²⁺: +2 (reduction)
- Oxidising Agent: MnO₄⁻ (permanganate ion) as it gains electrons (is reduced).
Question C: Describe the colour change you would see in reactions b and c in Question 8.
Reaction b: Br2(aq) + 2KI(aq) → 2KBr(aq) + I2(aq)
Colour Change:
- Before Reaction: The solution contains Br₂, which has a reddish-brown colour.
- After Reaction: Formation of I₂ imparts a yellow-brown colour to the solution.
Summary:
- Colour Change: Reddish-brown (Br₂) → Yellow-brown (I₂)
Reaction c: 5Fe2+(aq)+MnO4−(aq)+8H+(aq)→5Fe3+(aq)+Mn2+(aq)+4H2O(l)
Colour Change:
- Before Reaction: The solution contains MnO₄⁻ ions, which impart a purple colour.
- After Reaction: MnO₄⁻ is reduced to Mn²⁺, which is pale pink or colourless in solution.
Summary:
Colour Change: Purple (MnO₄⁻) → Pale Pink/Colourless (Mn²⁺)
Quizzes
Quiz 1