01.09 Chapter Summary
BioCast:
Kinetic Theory of Matter
- The Kinetic Theory of Matter explains the behavior of particles in different states of matter—solids, liquids, and gases—based on their arrangement and movement.
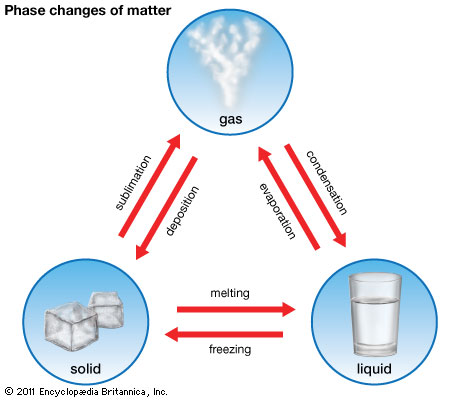
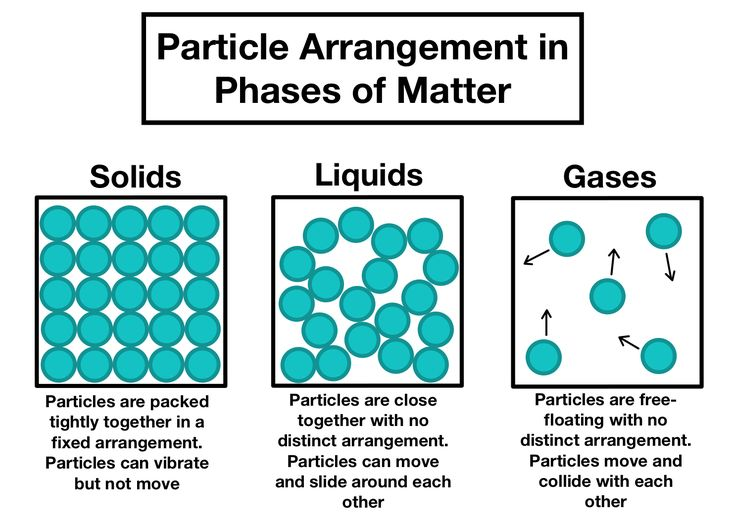
1. States of Matter
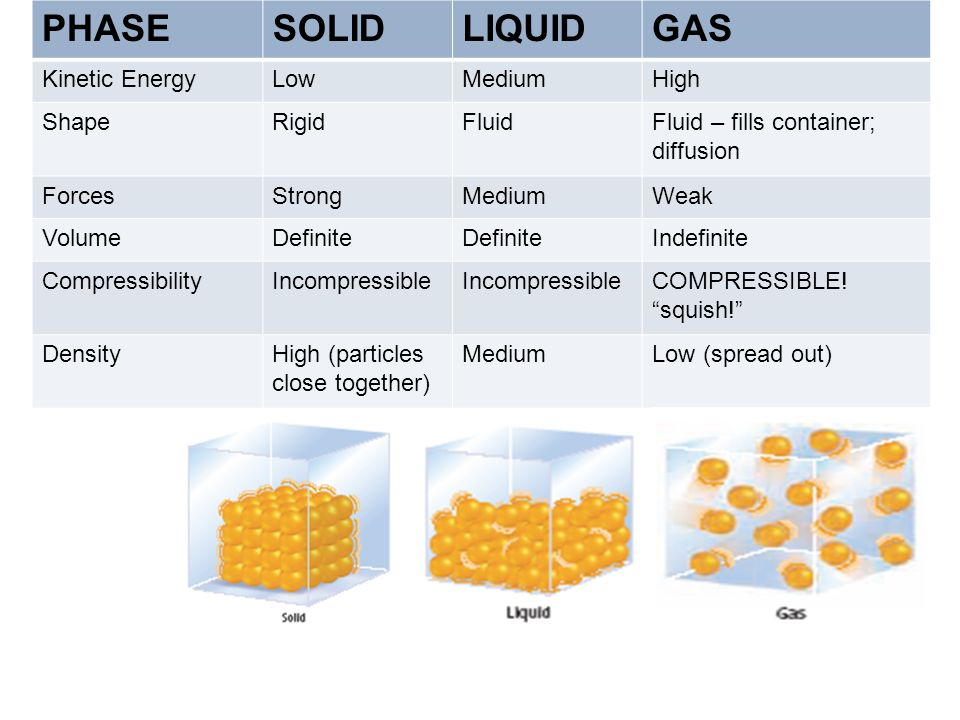
Solids
- Shape & Volume: Fixed shape and volume.
- Density: High density due to closely packed particles.
- Particle Movement: Particles vibrate in fixed positions; no movement from their places.
- Arrangement: Particles are arranged in a fixed, regular, and orderly pattern.
- Kinetic Particle Theory: Particles have low kinetic energy, restricting movement to vibrations.
Liquids
- Shape & Volume: Fixed volume but take the shape of their container.
- Density: Generally less dense than solids (water is an exception); much denser than gases.
- Particle Movement: Particles can move and slide past each other, allowing liquids to flow.
- Arrangement: Particles are close together but arranged randomly, not in a fixed pattern.
- Kinetic Particle Theory: Particles have greater kinetic energy than in solids, facilitating movement around each other.
Gases
- Shape & Volume: Neither fixed shape nor volume; they expand to fill their container.
- Density: Very low density.
- Particle Movement: Particles move rapidly and randomly in all directions (~500 m/s).
- Arrangement: Particles are far apart and can be easily compressed.
- Kinetic Particle Theory: Particles have the highest kinetic energy, enabling rapid and free movement.
2. State Changes
- State changes involve transitions between solid, liquid, and gas states, requiring changes in energy, arrangement, and particle movement.
- Melting (Solid → Liquid):
- Energy: Requires heat energy.
- Process: Heat transforms into kinetic energy, allowing particles to move freely.
- Temperature: Occurs at the melting point (e.g., water at 0°C).
- Freezing (Liquid → Solid):
- Energy: Releases heat energy.
- Process: Particles lose kinetic energy and arrange into a fixed pattern.
- Temperature: Occurs at the freezing point, same as the melting point.
- Boiling (Liquid → Gas):
- Energy: Requires heat energy.
- Process: Particles gain enough kinetic energy to form gas bubbles.
- Temperature: Occurs at the boiling point.
- Evaporation (Liquid → Gas):
- Energy: Requires kinetic energy from particles at the surface.
- Process: Occurs at the surface over a range of temperatures below the boiling point.
- Factors: Faster with larger surface area and higher temperatures.
- Condensation (Gas → Liquid):
- Energy: Releases heat energy.
- Process: Gas particles lose kinetic energy and come together to form a liquid.
- Temperature: Occurs over a range of temperatures as the gas cools.
Other:
- Sublimation:
- Sublimation is a phase transition process in which a substance transitions directly from a solid state to a gaseous state without passing through the intermediate liquid phase.
- Dry Ice (Solid CO₂): Sublimates at atmospheric pressure, turning directly into carbon dioxide gas without becoming liquid.
CO2 (solid)→CO2 (gas) - Iodine Crystals: Sublime upon heating, transitioning directly into iodine vapor.
- Dry Ice (Solid CO₂): Sublimates at atmospheric pressure, turning directly into carbon dioxide gas without becoming liquid.
- Sublimation is a phase transition process in which a substance transitions directly from a solid state to a gaseous state without passing through the intermediate liquid phase.
Kinetic Particle Theory Explanation:
- Melting and Boiling: Increase in kinetic energy allows particles to overcome intermolecular forces, transitioning to a more disordered state.
- Freezing and Condensation: Decrease in kinetic energy causes particles to lose movement freedom, allowing intermolecular forces to arrange particles into ordered structures.
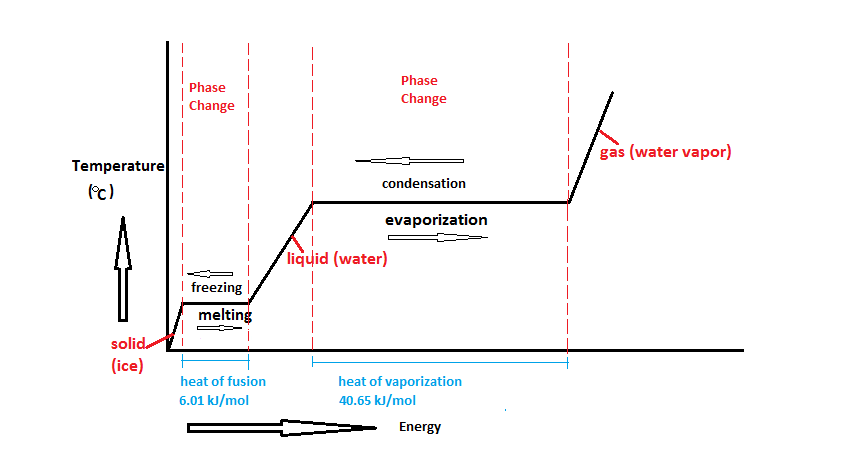
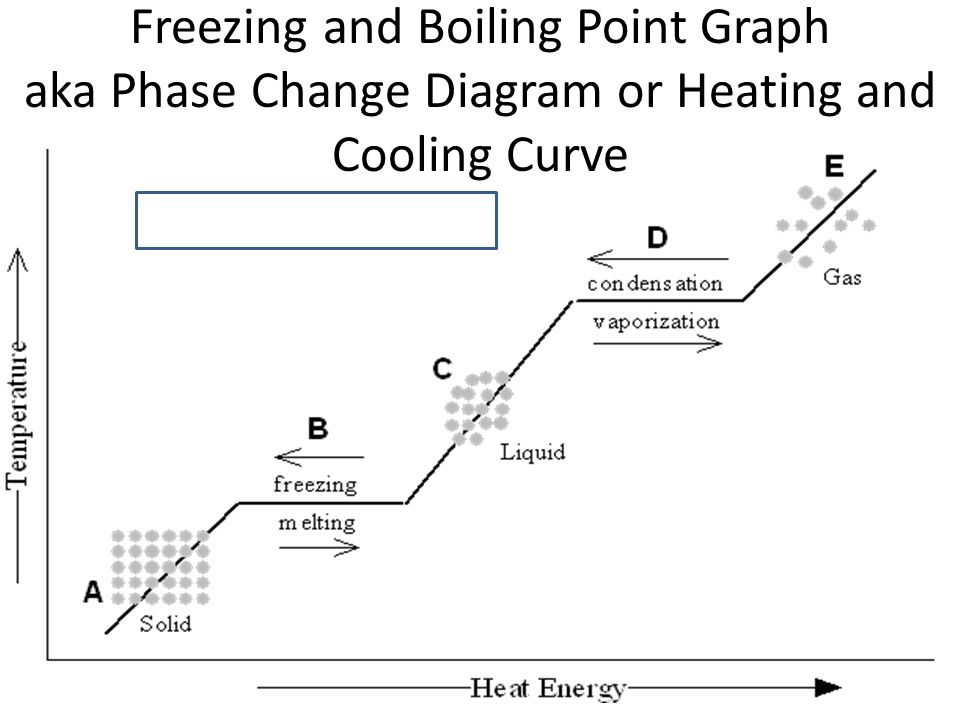
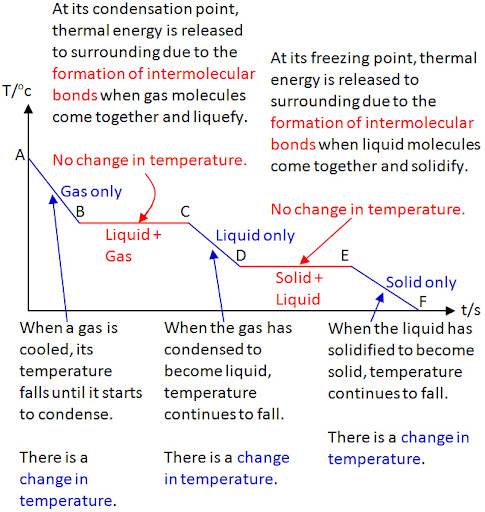
- Heating and Cooling Curves:
- Heating Curve: Shows temperature rise with plateaus during melting and boiling, indicating energy is used for state changes.
- Cooling Curve: Mirror image showing temperature decrease with plateaus during condensation and freezing.
3. Pressure and Temperature in Gases
- Temperature Effect:
- Increase: Gas volume expands if pressure is constant; density decreases (e.g., hot air balloon rises).
- Decrease: Gas volume contracts; density increases.
- Kinetic Particle Theory: Increasing temperature increases kinetic energy, causing particles to move faster and spread out.
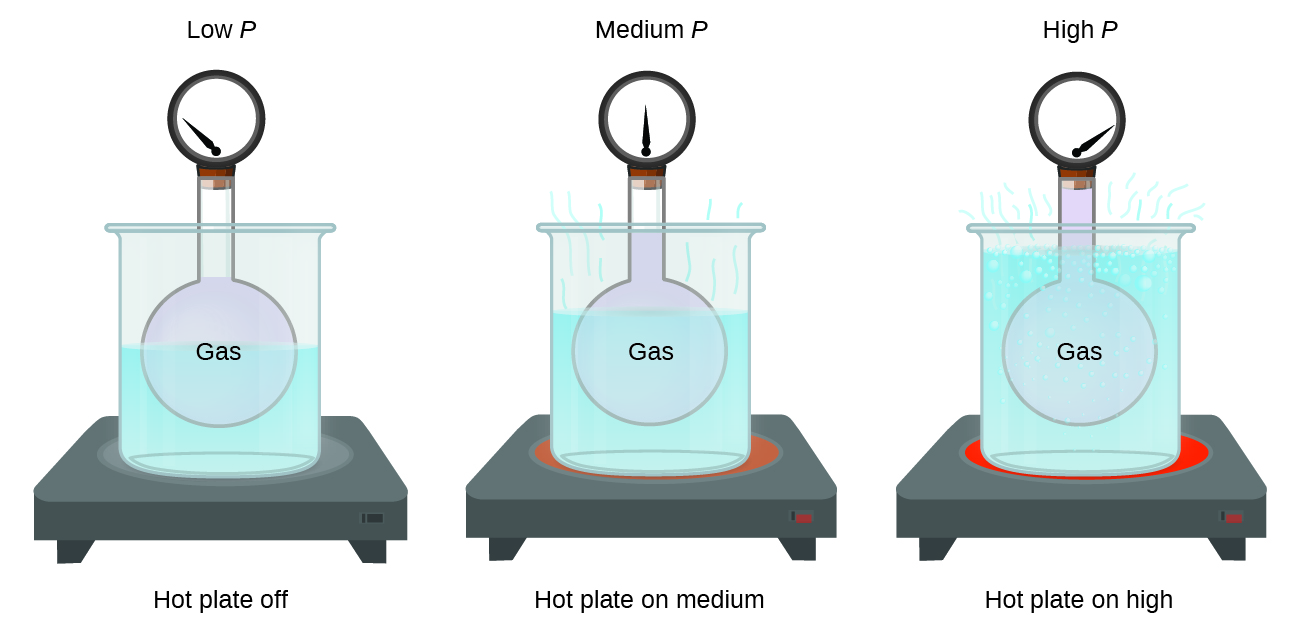
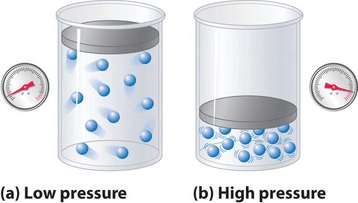
- Pressure Effect:
- Increased pressure: Gas volume decreases (if the temperature remains constant); particles collide more frequently with container walls (e.g., bicycle pump).
- Decreased pressure: Gas volume increases; particles collide less frequently.
- Kinetic Particle Theory: Increasing pressure means more particles occupy a smaller volume, leading to more frequent collisions.
Key Relationships:
- The symbol ∝ means “is proportional to.”
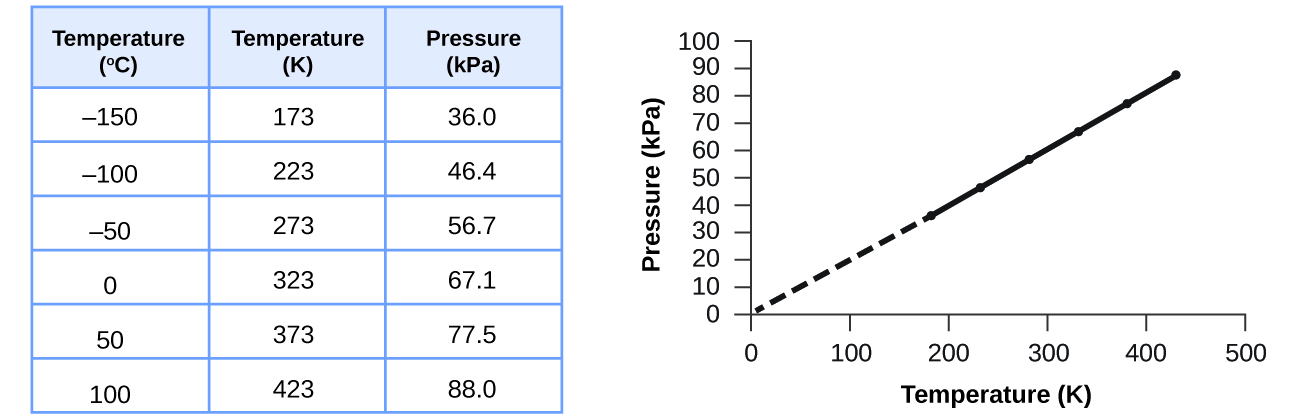
- Charles’s Law: Volume ∝ Temperature (at constant pressure).
- Boyle’s Law: Volume ∝ 1/Pressure (at constant temperature).
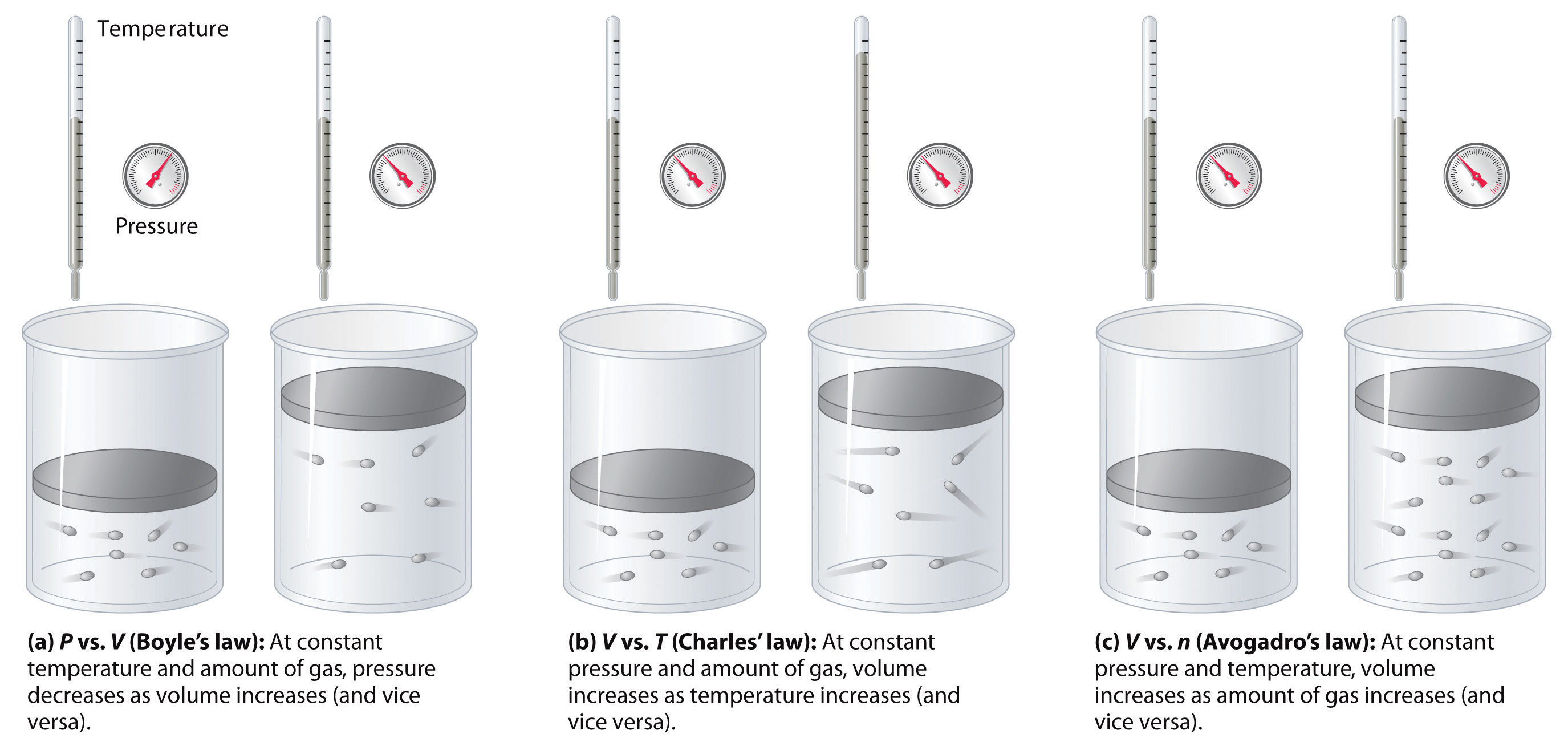
Real-Life Examples:
1. Hot Air Balloon
How It Works:
Heating the Air:
- Inside the balloon, there’s a burner that heats the air.
Air Expansion:
- When air is heated, its molecules move faster and spread out more. This means the air takes up more space, increasing its volume.
Decreased Density:
- Density is how much mass (or weight) is packed into a given space.
- Hot air has lower density than the cooler air outside the balloon because the molecules are more spread out.
Buoyant Force:
- Less dense air inside the balloon makes it lighter than the surrounding cooler air.
- Gravity pulls everything down, but the lighter balloon wants to rise, creating a buoyant force that lifts the balloon into the sky.
2. Bicycle Pump
How It Works:
Compressing Air:
- When you push down on the pump handle, you compress the air inside the pump’s chamber.
Increasing Pressure:
- Compressing air means that the air molecules are forced closer together, which increases the air pressure inside the pump.
Inflating the Tyre:
- The high-pressure air is then forced through a valve and into the bicycle tyre.
- This increases the tyre’s internal pressure, making it firm and ready for riding.
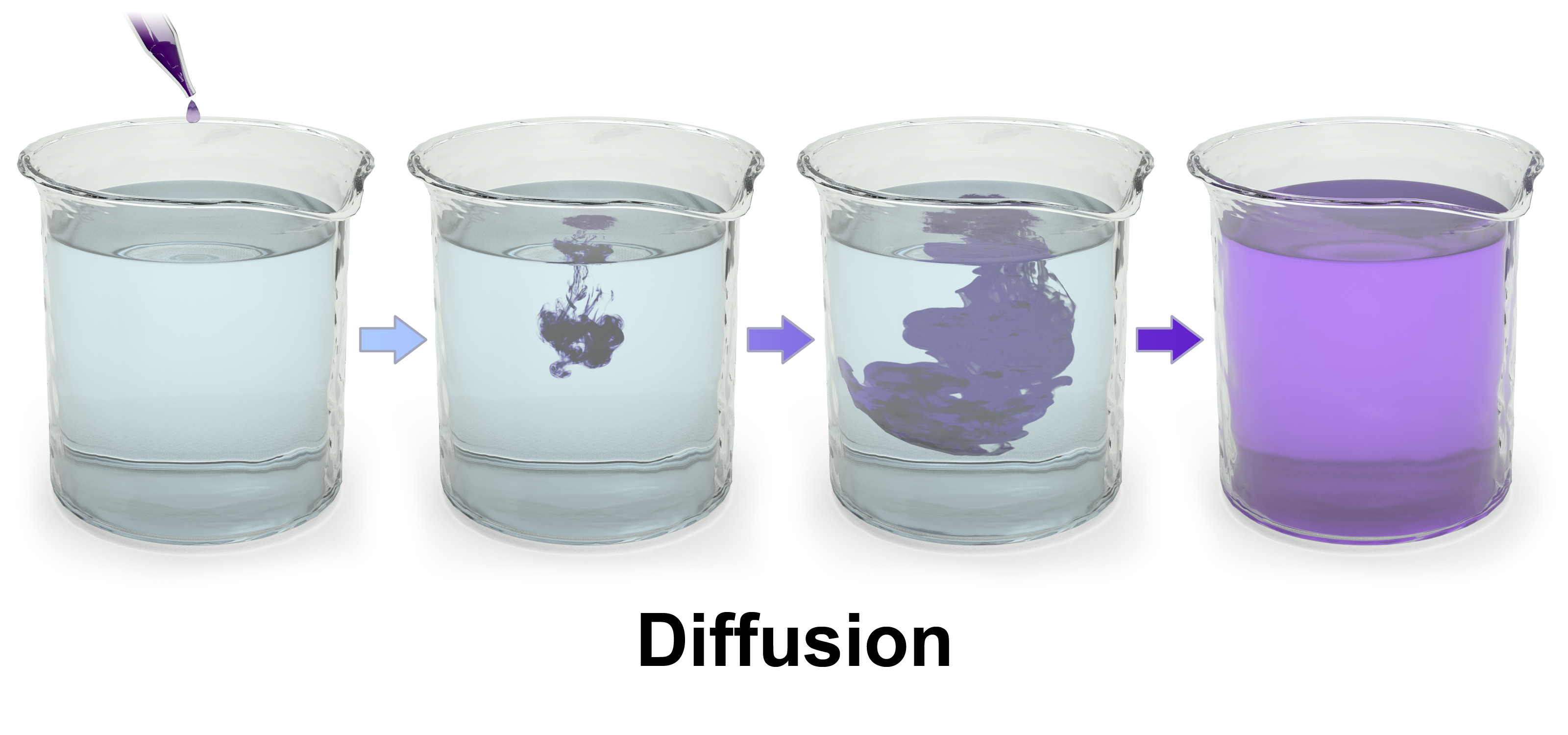
4. Diffusion
- Definition: Diffusion is the movement of particles from an area of high concentration to an area of low concentration until evenly distributed. It occurs in both gases and liquids due to the random motion of particles.
Explanation via Kinetic Particle Theory:
- Particle Movement: Continuous and random motion drives particles to spread out from regions of high concentration.
- Energy Consideration: Higher kinetic energy (e.g., higher temperatures) increases the rate of diffusion.
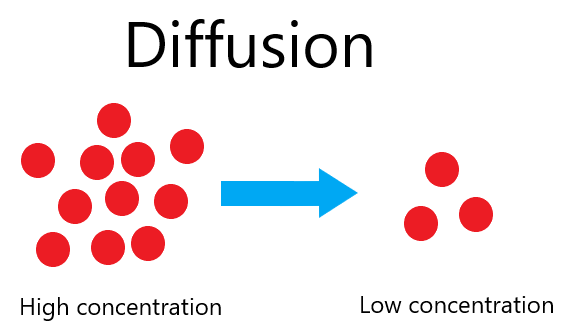
Factors Affecting Diffusion:
- Concentration Gradient: Greater difference speeds up diffusion.
- Temperature: Higher temperatures increase diffusion rates.
- Molecular Mass: Lower molecular mass gases diffuse faster.
Examples:
- In Liquids: Potassium manganate(VII) (KMnO₄) in water spreads evenly over time.
- In Gases: Bromine gas diffuses through air, forming a uniform concentration.
Quizzes
Quiz 1
Quiz 2
Practice Questions
Question 1: Multiple Choice
1. Which of the following statements correctly describes the arrangement of particles in a liquid?
A) Particles are arranged in a fixed, regular pattern.
B) Particles are far apart and move freely in all directions.
C) Particles are close together but arranged randomly.
D) Particles do not move and only vibrate in place.
Correct Answer: C) Particles are close together but arranged randomly.
Explanation:
- Option A: Describes solids, where particles are in a fixed, regular pattern.
- Option B: Describes gases, where particles are far apart and move freely.
- Option D: Also describes solids, where particles vibrate but do not move from their positions.
- Option C: Correctly describes liquids, where particles are close together but arranged randomly.
Question 2: Structured Question
2. (a) Draw a diagram to illustrate the arrangement of particles in a solid, liquid, and gas. Label each state of matter.
(b) Briefly explain how particle movement differs between a solid and a gas.
Answer Summary:
(a) Diagram:
- Solid: Particles tightly packed in a fixed, regular, orderly pattern with only small vibrations.
- Liquid: Particles close together but arranged randomly, able to move past one another.
- Gas: Particles far apart moving rapidly in all directions.
Note: Diagrams should clearly show the relative positions and movement of particles in each state.
(b) Explanation:
- Solid: Particles vibrate in fixed positions and do not move from their places.
- Gas: Particles move rapidly and randomly in all directions, freely expanding to fill the container.
Mark Allocation:
- Diagram: Up to 3 marks (1 mark per state if correctly drawn and labeled).
- Explanation: 2 marks (1 mark for each correct description of particle movement).
Total: 5 marks.
Question 3: Multiple Choice
3. Which state change involves particles gaining enough kinetic energy to form gas bubbles within a liquid?
A) Melting
B) Freezing
C) Boiling
D) Condensation
Answer Summary:
Correct Answer: C) Boiling
Explanation:
- Melting: Solid to liquid.
- Freezing: Liquid to solid.
- Boiling: Liquid to gas, involving formation of gas bubbles.
- Condensation: Gas to liquid.
Mark Allocation:
- 1 mark for selecting the correct option.
Question 4: Structured Question
4. (a) Explain Charles’s Law in terms of the relationship between temperature and volume of a gas at constant pressure.
(b) Provide a real-life example illustrating Charles’s Law.
Answer Summary:
(a) Explanation:
- Charles’s Law states that the volume of a gas is directly proportional to its temperature (in Kelvin) when pressure is constant. As temperature increases, volume increases; as temperature decreases, volume decreases.
(b) Real-Life Example:
- Hot Air Balloon: Heating the air inside the balloon increases its volume, making it less dense than the cooler air outside, causing the balloon to rise.
Mark Allocation:
- (a):2 marks
- 1 mark for stating the direct proportionality between volume and temperature.
- 1 mark for explaining that volume increases with temperature.
- (b): 1 mark for a correct real-life example.
Total: 3 marks.
Question 5: Multiple Choice
5. During which state change is heat energy released?
A) Melting
B) Evaporation
C) Condensation
D) Boiling
Answer Summary:
Correct Answer: C) Condensation
Explanation:
- Melting: Absorbs heat energy (solid to liquid).
- Evaporation: Absorbs heat energy (liquid to gas).
- Condensation: Releases heat energy (gas to liquid).
- Boiling: Absorbs heat energy (liquid to gas).
Mark Allocation:
- 1 mark for selecting the correct option.
Question 6: Structured Question
6. Describe how increasing the pressure on a gas affects its volume and particle collisions, according to the Kinetic Particle Theory.
Answer Summary:
- Volume Decreases: Increasing pressure compresses the gas into a smaller volume.
- More Frequent Collisions: Particles are closer together, leading to more frequent collisions with the container walls.
- Kinetic Particle Theory Explanation: Higher pressure means more particles occupy a smaller space, increasing the rate of collisions.
Mark Allocation:
- 2 marks for correctly describing the effect on volume.
- 2 marks for correctly explaining the effect on particle collisions and relating it to Kinetic Particle Theory.
Total: 4 marks.
Question 7: Multiple Choice
7. Which factor does NOT affect the rate of diffusion of a gas?
A) Temperature
B) Concentration gradient
C) Molecular mass
D) Volume of the container
Answer Summary:
- Correct Answer: D) Volume of the container
- Explanation:
- Temperature, concentration gradient, and molecular mass all affect the rate of diffusion.
- Volume of the container does not directly affect the rate at which particles diffuse.
- Mark Allocation:
- 1 mark for selecting the correct option.
Question 8: Structured Question
8. (a) What happens to the kinetic energy of particles during the freezing of water?
(b) How does this relate to the arrangement of particles in the solid state?
Answer Summary:
(a) Kinetic Energy:
- Kinetic energy decreases as particles lose energy during freezing.
(b) Relation to Arrangement:
- Lower kinetic energy causes particles to arrange into a fixed, orderly pattern, forming a solid structure.
Mark Allocation:
- (a): 1 mark for stating that kinetic energy decreases.
- (b): 2 marks for explaining the relationship between decreased kinetic energy and the fixed, orderly arrangement of particles.
Total: 3 marks.
Question 9: Multiple Choice
9. Which of the following best explains why lighter gases diffuse faster than heavier gases?
A) Lighter gases have higher density.
B) Lighter gases have higher kinetic energy at a given temperature.
C) Lighter gases have lower kinetic energy at a given temperature.
D) Lighter gases occupy more volume.
Answer Summary:
Correct Answer: B) Lighter gases have higher kinetic energy at a given temperature.
Explanation:
- Lighter gases have lower molecular mass, leading to higher speeds and higher kinetic energy at the same temperature, thus diffusing faster.
- Option A: Incorrect; lighter gases typically have lower density.
- Option C: Incorrect; they have higher, not lower, kinetic energy.
- Option D: Incorrect; diffusion rate is not directly about volume occupied.
Mark Allocation:
- 1 mark for selecting the correct option.
Question 10: Structured Question
10. (a) Explain why a hot air balloon rises using the concepts of kinetic particle theory.
(b) Describe what happens to the density of the air inside the balloon compared to the cooler air outside.
Answer Summary:
(a) Explanation:
- Heating the air inside the balloon increases the kinetic energy of the particles, causing them to move faster and spread apart, increasing the volume of the air.
(b) Density Description:
- Increased volume leads to lower density of the heated air inside the balloon compared to the cooler, denser air outside, causing the balloon to rise (just like an air bubble in water, CO2 gas bubbles in cola).
Mark Allocation:
- (a):2 marks
- 1 mark for linking heating to increased kinetic energy.
- 1 mark for explaining how increased kinetic energy causes particles to spread apart and increase volume.
- (b):2 marks
- 1 mark for stating that the density inside decreases.
- 1 mark for com paring it to the denser cooler air outside.
Total: 4 marks.
Question 11: Multiple Choice
11. Which of the following best describes the kinetic energy of particles in a gas compared to those in a solid?
A) Particles in a gas have less kinetic energy than those in a solid.
B) Particles in a gas have the same kinetic energy as those in a solid.
C) Particles in a gas have more kinetic energy than those in a solid.
D) Kinetic energy of particles does not depend on the state of matter.
Correct Answer: C) Particles in a gas have more kinetic energy than those in a solid.
Explanation:
- Option A: Incorrect; gas particles have more kinetic energy.
- Option B: Incorrect; kinetic energy varies with state.
- Option D: Incorrect; kinetic energy depends on the state and temperature.
- Option C: Correct as gas particles move freely and rapidly, indicating higher kinetic energy compared to the vibrating particles in a solid.
Mark Allocation:
- 1 mark for selecting the correct option.
Question 12: Structured Question
12. (a) Describe what occurs to the kinetic energy and arrangement of particles when water evaporates.
(b) How does temperature affect the rate of evaporation?
Answer Summary:
(a) Kinetic Energy and Arrangement:
- Kinetic Energy: Increases as particles gain energy to overcome intermolecular forces.
- Arrangement: Particles move from a close, random arrangement in liquid to a dispersed arrangement in gas.
(b) Effect of Temperature on Evaporation:
- Higher Temperature: Increases kinetic energy of particles, speeding up the rate of evaporation.
- Lower Temperature: Decreases kinetic energy, slowing down evaporation.
Mark Allocation:
- (a):2 marks
- 1 mark for describing the increase in kinetic energy.
- 1 mark for explaining the change in arrangement from liquid to gas.
- (b):2 marks
- 1 mark for linking higher temperature to increased kinetic energy.
- 1 mark for stating that increased kinetic energy speeds up evaporation.
Total: 4 marks.
Question 13: Multiple Choice
13. Which state change occurs directly from solid to gas without passing through the liquid state?
A) Melting
B) Freezing
C) Sublimation
D) Condensation
Correct Answer: C) Sublimation
Explanation:
- Melting: Solid to liquid.
- Freezing: Liquid to solid.
- Sublimation: Solid to gas directly.
- Condensation: Gas to liquid.
Mark Allocation:
- 1 mark for selecting the correct option.
Question 14: Structured Question
14. (a) Explain how Boyle’s Law relates pressure and volume of a gas.
(b) Calculate the new volume of a gas when its pressure increases from 1.00 atm to 2.00 atm, assuming the temperature remains constant and the initial volume was 3.00 L.
Answer Summary:
(a) Explanation of Boyle’s Law:
- Boyle’s Law states that the volume of a given mass of gas is inversely proportional to its pressure when temperature is constant.
- Mathematically:
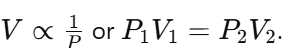
(b) Calculation:
Given:
- Initial Pressure, P1 = 1.00 atm
- Initial Volume, V1 = 3.00 L
- Final Pressure, P2 = 2.00 atm
Using Boyle’s Law:
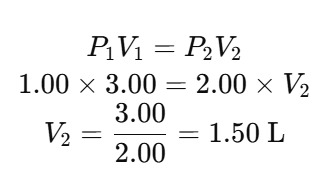
Mark Allocation:
- (a):2 marks
- 1 mark for stating the inverse relationship.
- 1 mark for the correct mathematical expression.
- (b):3 marks
- 1 mark for setting up the equation.
- 1 mark for correct substitution.
- 1 mark for accurate calculation and final answer.
Total: 5 marks.
Question 15: Multiple Choice
15. Which of the following best illustrates diffusion?
A) Ice melting in a glass of water.
B) Perfume spreading through a room.
C) Water boiling in a kettle.
D) Sugar dissolving in tea.
Correct Answer: B) Perfume spreading through a room.
Explanation:
- Option A: Melting involves a state change from solid to liquid.
- Option B: Perfume molecules spreading out in the air is a classic example of diffusion.
- Option C: Boiling involves a state change from liquid to gas.
- Option D: Sugar dissolving involves solute particles dispersing in a solvent but is better described as dissolution rather than diffusion in the context of particle movement from high to low concentration.
Mark Allocation:
- 1 mark for selecting the correct option.
Question 16: Structured Question
16. (a) Describe what occurs during the cooling curve of water as it changes from gas to liquid and then to solid.
(b) Label the plateaus on the cooling curve and explain what they represent.
Note: Include a diagram of a cooling curve showing temperature vs. energy with plateaus.
Answer Summary:
(a) Description of Cooling Curve:
- As water cools from gas to liquid:
- Kinetic Energy: Decreases as particles lose energy.
- State Change: Gas to liquid (condensation).
- As water further cools from liquid to solid:
- Kinetic Energy: Continues to decrease.
- State Change: Liquid to solid (freezing).
(b) Labeling Plateaus:
- First Plateau: Represents the temperature at which condensation occurs (gas to liquid). Energy is released during this phase change despite cooling.
- Second Plateau: Represents the temperature at which freezing occurs (liquid to solid). Energy is released as the substance changes state.
Mark Allocation:
- (a):3 marks
- 1 mark for describing the decrease in kinetic energy.
- 1 mark for explaining the gas to liquid transition.
- 1 mark for explaining the liquid to solid transition.
- (b):2 marks
- 1 mark for correctly labeling each plateau.
- 1 mark for explaining what each plateau represents.
Total: 5 marks.
Question 17: Multiple Choice
17. Which of the following correctly matches the state of matter with its density?
A) Solid – Low density
B) Liquid – High density
C) Gas – Medium density
D) Solid – High density
Correct Answer: D) Solid – High density
Explanation:
- Option A: Incorrect; solids typically have high density.
- Option B: Partially incorrect; while some liquids have high density (e.g., water), generally, liquids have lower density than solids.
- Option C: Incorrect; gases have very low density.
- Option D: Correct as solids generally have high density due to closely packed particles.
Mark Allocation:
- 1 mark for selecting the correct option.
Question 18: Structured Question
18. Explain how increasing the temperature of a gas affects its pressure, assuming the volume remains constant. Use the Kinetic Particle Theory in your explanation.
Answer Summary:
Effect of Increasing Temperature:
- Kinetic Energy: Increasing temperature raises the kinetic energy of gas particles.
- Particle Movement: Particles move faster and collide more forcefully and more frequently with the container walls.
- Resulting Pressure: This leads to an increase in pressure, as more force is exerted per unit area.
Kinetic Particle Theory Link:
- According to the Kinetic Particle Theory, higher kinetic energy from increased temperature causes particles to move faster, resulting in more frequent and forceful collisions, thereby increasing pressure when volume is constant.
Mark Allocation:
- 2 marks for correctly explaining the relationship between temperature and kinetic energy.
- 2 marks for linking increased kinetic energy to higher pressure through more frequent and forceful collisions.
Total: 4 marks.
Question 19: Multiple Choice
19. Which of the following best explains why solids have a fixed shape and volume?
A) Their particles have high kinetic energy and move freely.
B) Their particles are closely packed in a fixed, orderly arrangement and can only vibrate.
C) Their particles are far apart and move rapidly in all directions.
D) Their particles are randomly arranged and can slide past each other.
- Correct Answer: B) Their particles are closely packed in a fixed, orderly arrangement and can only vibrate.
- Explanation:
- Option A: Describes gases.
- Option B: Correctly describes solids.
- Option C: Describes gases.
- Option D: Describes liquids.
- Mark Allocation:
- 1 mark for selecting the correct option.
Question 20: Structured Question
20. (a) Compare the density and kinetic energy of particles in solids, liquids, and gases.
(b) How does density relate to the arrangement of particles in each state of matter?
Answer Summary:
(a) Comparison of Density and Kinetic Energy:
- Solids:
- Density: High
- Kinetic Energy: Low (particles vibrate in fixed positions)
- Liquids:
- Density: Medium
- Kinetic Energy: Moderate (particles can move and slide past each other)
- Gases:
- Density: Low
- Kinetic Energy: High (particles move rapidly and freely)
(b) Relation of Density to Particle Arrangement:
- Solids: Particles are closely packed in a fixed, orderly arrangement, leading to high density.
- Liquids: Particles are close together but arranged randomly, resulting in medium density.
- Gases: Particles are far apart and spread out, causing low density.
Mark Allocation:
(a):3 marks
- 1 mark for correctly stating the density of each state.
- 2 marks for accurately describing the kinetic energy of particles in each state.
(b):2 marks
- 1 mark for linking high density to closely packed, orderly arrangement in solids.
- 1 mark for explaining how random or spread-out arrangements in liquids and gases correspond to their densities.
- Total: 5 marks.