01.03 Pure Substances
1. Pure Substances
Definition
- Pure Substance: A substance that consists of only one type of chemical element or compound without any contaminating impurities. It has specific and consistent properties, including definite melting and boiling points.
Characteristics of Pure Substances
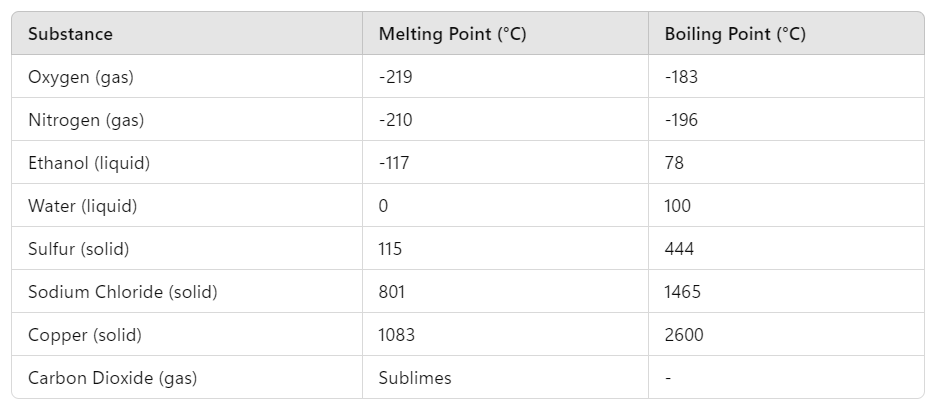
- Definite Melting and Boiling Points: Pure substances melt and boil at precise temperatures under atmospheric pressure. These points are consistent and predictable, which helps in identifying and verifying the purity of a substance.Table 1.2: Melting and Boiling Points of Common Substances at Atmospheric Pressure
Key Terms
- Melting Point: The temperature at which a solid becomes a liquid.
- Boiling Point: The temperature at which a liquid becomes a gas (boils).
- Sublime: The transition of a substance directly from a solid to a gas without passing through the liquid state (e.g., carbon dioxide).
Applications of Melting and Boiling Points
- Purity Testing: Since pure substances have specific melting and boiling points, measuring these can confirm the purity of a sample.
- Identification of Substances: Comparing the melting and boiling points of an unknown substance with known values helps in identifying it.
- Determining States of Matter at Room Temperature (25°C):
- Solid: If both melting and boiling points are above 25°C.
- Liquid: If the melting point is below 25°C and the boiling point is above 25°C.
- Gas: If both melting and boiling points are below 25°C.
- Water: Melting point = 0°C, Boiling point = 100°C → Liquid at room temperature.
- Ethanol: Melting point = -117°C, Boiling point = 78°C → Liquid at room temperature.
- Nitrogen: Melting point = -210°C, Boiling point = -196°C → Gas at room temperature.
2. States of Matter
Solid, Liquid, and Gas
- Solid: Definite shape and volume. Particles are closely packed in a fixed arrangement.
- Liquid: Definite volume but takes the shape of its container. Particles are less tightly packed than in solids and can move past each other.
- Gas: Neither definite shape nor volume. Particles are widely spaced and move freely.
Effect of Temperature on States of Matter
- Heating a Substance: Increases the kinetic energy of particles, potentially changing the state from solid to liquid (melting) or liquid to gas (boiling).
- Cooling a Substance: Decreases the kinetic energy of particles, potentially changing the state from gas to liquid (condensation) or liquid to solid (freezing).
3. Effect of Impurities
Impurities in Substances
- Impure Substance: Contains one or more different substances mixed with the pure substance.
- Example: Seawater is impure water containing dissolved salts and other minerals.
Impact on Melting and Boiling Points
- Depression of Melting Point: Impurities can lower the melting point of a substance.
- Elevation of Boiling Point: Impurities can raise the boiling point of a substance.
- Melting and Boiling Over a Range: Unlike pure substances, impure substances may melt or boil over a range of temperatures rather than at a precise point.Example:
- Seawater:
- Freezing Point: Below 0°C (pure water freezes at 0°C).
- Boiling Point: Above 100°C (pure water boils at 100°C).
- Seawater:
Demonstration of Impurities
- Evaporation of Seawater: Heating seawater causes water to evaporate, leaving behind salt as a solid residue.
Figure 1.7: Solid salt formations on the surface of the Dead Sea after evaporation.
4. Practical Applications and Examples
Testing Purity
- Melting-Point Apparatus: An electrically heated device used to measure the melting point of a solid.
- Procedure:
- Place a small sample of the substance in a capillary tube.
- Insert the tube into the melting-point apparatus.
- Gradually increase the temperature and observe the sample.
- Record the temperature at which the substance starts to melt and when it completely liquefies.
Identifying Unknown Substances
- Comparison Method: Measure the melting and boiling points of the unknown substance and compare them with known values.Example:
- If an unknown substance melts at 0°C and boils at 100°C, it is likely pure water.
5. Practice Questions
Question 1
State the names for the following physical changes:
a) Liquid to Solid
Answer: Freezing
Example: Water turning into ice.
b) Liquid to Gas at a Precise Temperature
Answer: Boiling
Example: Water boiling at 100°C.
c) Gas to Liquid
Answer: Condensation
Example: Water vapor condensing into liquid water on a cold surface.
Question 2
The melting and boiling points of three pure substances are given in Table 1.3.
| Substance | Melting Point (°C) | Boiling Point (°C) |
|---|---|---|
| Ethanol | -117 | 78 |
| Methane | -182 | -164 |
| Mercury | -30 | 357 |
a) Which of them has the lowest melting point?
Answer: Methane
Explanation: Methane has the lowest melting point at -182°C.
b) Which two substances are liquids at room temperature? Explain your answer.
Answer: Ethanol and Mercury
Explanation:
- Ethanol: Melting point = -117°C, Boiling point = 78°C.
At room temperature (25°C), ethanol is a liquid because its melting point is below 25°C and boiling point is above 25°C. - Mercury: Melting point = -30°C, Boiling point = 357°C.
At room temperature, mercury is a liquid for the same reason.
c) What effect does the presence of an impurity have on the freezing point of a liquid?
Answer: It Lowers the Freezing Point
Explanation: Impurities disrupt the orderly arrangement of molecules, making it harder for the substance to solidify, thus lowering its freezing point.
6. Summary
- Pure substances have specific and consistent melting and boiling points, useful for identifying and testing purity.
- States of matter (solid, liquid, gas) are determined by temperature relative to melting and boiling points.
- Impurities alter melting and boiling points, often causing substances to melt or boil over a range of temperatures rather than at precise points.
- Practical techniques, such as using a melting-point apparatus, aid in the identification and purity testing of substances.
- Understanding these concepts is fundamental for various applications in chemistry, including material synthesis, quality control, and environmental studies.