02.05 Carbohydrates
Carbohydrates: Monosaccharides
Carbohydrates are organic molecules containing carbon, hydrogen, and oxygen atoms, with a typical 2:1 hydrogen to oxygen ratio (as in water). The general formula for carbohydrates is Cx(H2O)y.

Types of Carbohydrates
Carbohydrates are divided into three main groups:
- Monosaccharides – Single sugar molecules
- Disaccharides – Two sugar molecules linked together
- Polysaccharides – Long chains of sugar molecules
Monosaccharides
- Definition: Simple sugars that dissolve in water, forming sweet-tasting solutions.
- General Formula: (CH2O)n
- Classification by Carbon Atoms:
- Trioses (3 carbons)
- Pentoses (5 carbons) – Examples: Ribose and Deoxyribose
- Hexoses (6 carbons) – Examples: Glucose, Fructose, and Galactose
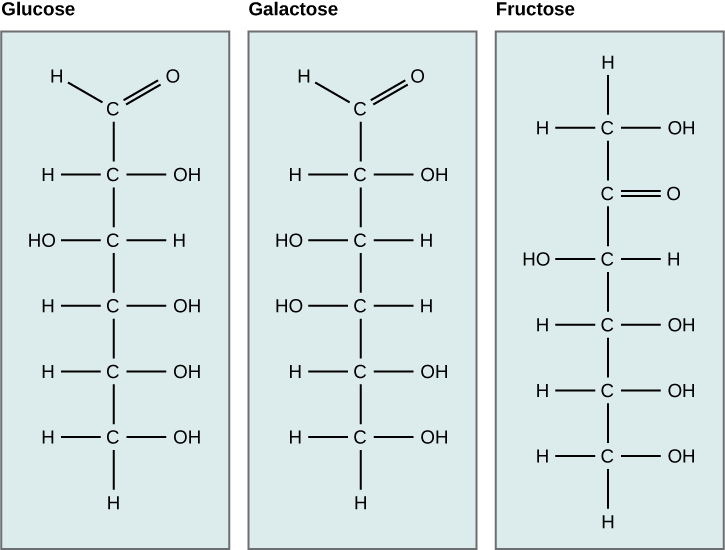
Molecular and Structural Formulae
- Hexose Example: Glucose (C6H12O6)
- Molecular formula shows 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms.
- Glucose can be represented in both straight-chain and ring structures.
Ring Structures and Isomers
- Ring Formation: In pentoses and hexoses, the carbon chain can close to form a stable ring structure.
- Glucose Ring Formation: Carbon atom 1 bonds to the oxygen on carbon atom 5, forming a ring.
- Isomers of Glucose:
- α-Glucose (hydroxyl group on carbon 1 below the ring)
- β-Glucose (hydroxyl group on carbon 1 above the ring)
- Isomers have significant biological roles in forming structures like starch, glycogen, and cellulose.
Functions of Monosaccharides in Living Organisms
- Energy Source:
- Monosaccharides, especially glucose, are crucial for cellular respiration.
- High energy is released by breaking carbon-hydrogen bonds, which helps produce ATP from ADP and phosphate.
- Building Blocks for Larger Molecules:
- Pentoses (ribose and deoxyribose) are components of RNA, ATP, and DNA.
- Glucose is the building block for polysaccharides like starch, glycogen, and cellulose.
Carbohydrates: Disaccharides
Disaccharides are sugars formed by linking two monosaccharides through a glycosidic bond.
Common Disaccharides
Maltose: Glucose + Glucose
- Found in germinating seeds.
Sucrose: Glucose + Fructose
- Primary transport sugar in plants and the common table sugar.
Lactose: Glucose + Galactose
- Major sugar in milk, crucial for the diet of young mammals.
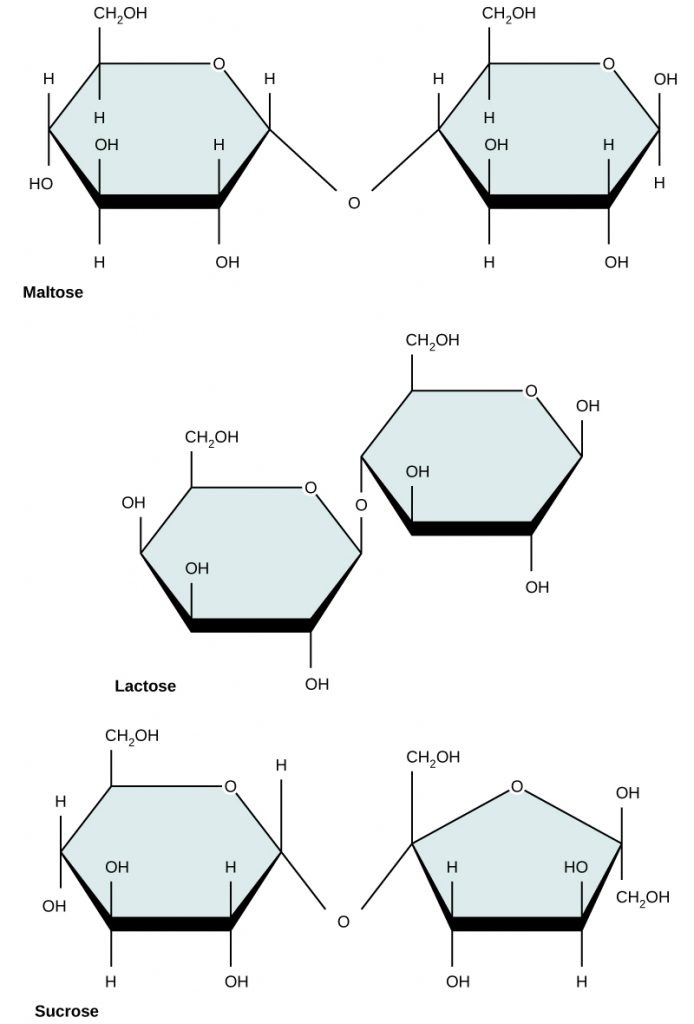
Formation and Breakdown of Disaccharides
- Condensation Reaction:
- Disaccharides form when two monosaccharides join by removing a water molecule.
- Two hydroxyl (–OH) groups align; one hydroxyl donates an H atom, forming H2O.
- An oxygen bridge (C–O–C link) forms between the two sugar molecules, creating a glycosidic bond.
- Hydrolysis Reaction:
- Disaccharides can be split into monosaccharides by adding water (hydrolysis).
- Hydrolysis occurs during digestion, breaking down disaccharides into simpler sugars.
Key Concepts
Glycosidic Bond:
- A C–O–C bond linking two sugar molecules.
- A type of covalent bond formed via condensation.
Enzyme-Specific Bond Formation:
- Enzymes determine which hydroxyl groups form the glycosidic bond.
- Though monosaccharides have multiple –OH groups, only specific glycosidic linkages are common in nature due to enzyme activity.
Carbohydrates: Polysaccharides
Polysaccharides are large polymers made by linking many monosaccharides (sugar molecules) through condensation reactions that create glycosidic bonds. Each chain may contain thousands of monosaccharide units, forming macromolecules.
- Key Polysaccharides:
- Starch (plants)
- Glycogen (animals)
- Cellulose (plants)
Functions and Storage Forms of Polysaccharides
- Energy Storage:
- Glucose serves as a primary energy source, but storing it directly would disrupt cell osmotic balance and interfere with cell functions.
- Organisms convert glucose into insoluble, compact polysaccharides to avoid these issues.
- Plants store glucose as starch, while animals store it as glycogen.
- Breakdown for Energy:
- Polysaccharides are broken down into glucose when energy is needed, through enzyme-controlled hydrolysis.
Starch (Plant Storage Polysaccharide)
- Components of Starch:
- Amylose:
- Formed from α-glucose molecules with 1,4 glycosidic bonds.
- Structure: Unbranched, helical chains that coil into compact shapes.
- Amylopectin:
- Consists of α-glucose with 1,4 glycosidic bonds and 1,6 linkages that create branches.
- Branching allows quicker release of glucose during hydrolysis.
- Starch Granules:
- Starch forms large granules found in chloroplasts and storage organs (e.g., potato tubers).
- Starch grains are visible under a microscope when stained with iodine-potassium iodide solution.
- Amylose:
Glycogen (Animal Storage Polysaccharide)
- Structure of Glycogen:
- Similar to amylopectin but more highly branched.
- Composed of α-glucose units with 1,4 and 1,6 glycosidic bonds.
- High branching allows rapid release of glucose to meet high energy demands in animals.
- Glycogen Granules:
- Glycogen clumps together to form granules visible in liver and muscle cells, acting as an energy reserve.

Practise Questions 1
Questions
1. What type of chemical reaction happens when glucose is formed from starch or glycogen?
- Answer: Hydrolysis reaction, which breaks down polysaccharides by adding water, releasing glucose.
2. List five ways in which the molecular structures of glycogen and amylopectin are similar.
Answer:
- Both are branched polysaccharides.
- Both serve as energy storage molecules in cells.
- Both are made from α-glucose monomers.
- Both have 1,4 glycosidic bonds in the main chain.
- Both contain 1,6 glycosidic bonds that create branch points.
Practise Questions 2
Question 1
What are monosaccharides? Describe their general formula, classification, and provide examples. (6 marks)
Mark Scheme:
- Monosaccharides are simple sugars that dissolve in water to form sweet-tasting solutions. (1 mark)
- General formula: (CH₂O)n, where nnn is typically 3-7. (1 mark)
- Classified by the number of carbon atoms:
- Trioses (3 carbons)
- Pentoses (5 carbons, e.g., ribose, deoxyribose)
- Hexoses (6 carbons, e.g., glucose, fructose, galactose). (1 mark)
- Example: Glucose (C₆H₁₂O₆) is a hexose sugar. (1 mark)
- Monosaccharides are the building blocks for disaccharides and polysaccharides. (1 mark)
- They serve as a primary energy source for cellular respiration. (1 mark)
Question 2
Explain the structure and significance of α-glucose and β-glucose isomers. (6 marks)
Mark Scheme:
- Glucose can form two isomers: α-glucose and β-glucose, depending on the position of the hydroxyl group (-OH) on carbon 1. (1 mark)
- In α-glucose, the -OH group is below the plane of the ring. (1 mark)
- In β-glucose, the -OH group is above the plane of the ring. (1 mark)
- α-glucose forms storage polysaccharides like starch and glycogen. (1 mark)
- β-glucose forms structural polysaccharides like cellulose, which provides rigidity to plant cell walls. (1 mark)
- The distinct roles of these isomers illustrate the importance of molecular structure in biological functions. (1 mark)
Question 3
Describe the formation of disaccharides, including the role of glycosidic bonds. (6 marks)
Mark Scheme:
- Disaccharides form through a condensation reaction, where two monosaccharides join and a molecule of water is removed. (1 mark)
- The bond formed is a glycosidic bond (C–O–C). (1 mark)
- Example 1: Glucose + Glucose → Maltose. (1 mark)
- Example 2: Glucose + Fructose → Sucrose. (1 mark)
- Enzymes control which hydroxyl (-OH) groups participate in the bond formation, ensuring specificity. (1 mark)
- Disaccharides can be broken down into monosaccharides via hydrolysis, adding water to break the glycosidic bond. (1 mark)
Question 4
Compare starch and glycogen in terms of structure and function. (6 marks)
Mark Scheme:
- Starch is the primary storage polysaccharide in plants, composed of amylose and amylopectin. (1 mark)
- Amylose: Unbranched helical chains with 1,4 glycosidic bonds. (1 mark)
- Amylopectin: Branched chains with 1,4 and 1,6 glycosidic bonds. (1 mark)
- Glycogen is the primary storage polysaccharide in animals, similar to amylopectin but more highly branched. (1 mark)
- Branching in glycogen allows faster release of glucose during hydrolysis, meeting animals’ high energy demands. (1 mark)
- Both are insoluble, preventing osmotic imbalance, and serve as compact energy reserves. (1 mark)
Question 5
Explain the structure and function of cellulose. (6 marks)
Mark Scheme:
- Cellulose is a polysaccharide made of β-glucose units linked by 1,4 glycosidic bonds. (1 mark)
- Each β-glucose unit is rotated 180°, allowing straight, unbranched chains. (1 mark)
- Chains form hydrogen bonds with adjacent chains, creating strong microfibrils. (1 mark)
- Function: Provides structural support in plant cell walls. (1 mark)
- Its strength and rigidity prevent cell collapse under osmotic pressure. (1 mark)
- Cellulose is indigestible to humans but forms dietary fiber, aiding digestion. (1 mark)
Question 6
What is the role of monosaccharides as energy sources and building blocks? (6 marks)
Mark Scheme:
- Monosaccharides like glucose are primary energy sources for cellular respiration. (1 mark)
- Breaking carbon-hydrogen bonds in glucose releases energy to produce ATP. (1 mark)
- They act as building blocks for larger molecules:
- Glucose forms polysaccharides (e.g., starch, glycogen, cellulose). (1 mark)
- Pentoses like ribose are components of RNA, ATP, and DNA. (1 mark)
- Monosaccharides dissolve easily in water, making them readily transportable in the bloodstream. (1 mark)
- Their versatility underpins their central role in metabolism. (1 mark)
Question 7
Describe how polysaccharides are adapted for storage and energy release. (5 marks)
Mark Scheme:
- Polysaccharides like starch and glycogen are compact, allowing efficient storage. (1 mark)
- They are insoluble, preventing osmotic effects in cells. (1 mark)
- Starch (plants) and glycogen (animals) are highly branched, enabling rapid hydrolysis by enzymes. (1 mark)
- Hydrolysis releases glucose monomers, which can be used in cellular respiration for energy. (1 mark)
- These adaptations make polysaccharides efficient energy reserves. (1 mark)
Question 8
Explain the significance of glycosidic bonds in carbohydrate structure and function. (6 marks)
Mark Scheme:
- Glycosidic bonds are covalent bonds formed between monosaccharides during condensation reactions. (1 mark)
- These bonds determine the structure of polysaccharides:
- 1,4 bonds create linear chains (e.g., amylose, cellulose). (1 mark)
- 1,6 bonds create branching (e.g., amylopectin, glycogen). (1 mark)
- The structure affects the polysaccharide’s function:
- Branched structures allow rapid energy release.
- Linear structures provide rigidity and support. (1 mark)
- Glycosidic bonds can be broken by hydrolysis, releasing monosaccharides for energy use. (1 mark)
- Enzyme specificity ensures precise formation and breakdown of glycosidic bonds. (1 mark)
Question 9
What are the differences between α-glucose and β-glucose, and how do these affect polysaccharide formation? (6 marks)
Mark Scheme:
- In α-glucose, the hydroxyl group (-OH) on carbon 1 is below the ring, while in β-glucose, it is above the ring. (1 mark)
- α-glucose forms polysaccharides like starch and glycogen, which are storage molecules. (1 mark)
- β-glucose forms polysaccharides like cellulose, which provide structural support. (1 mark)
- The position of the -OH group in β-glucose allows 180° rotation, enabling hydrogen bonding between chains. (1 mark)
- This difference in bonding makes cellulose strong and indigestible to most animals, unlike starch and glycogen. (1 mark)
- These structural differences illustrate the significance of molecular isomerism in biological functions. (1 mark)
Question 10
How do condensation and hydrolysis reactions contribute to carbohydrate metabolism? (6 marks)
Mark Scheme:
- Condensation reactions link monosaccharides into disaccharides and polysaccharides, storing energy. (1 mark)
- Example: Glucose + Glucose → Maltose + Water. (1 mark)
- Hydrolysis reactions break down polysaccharides into monosaccharides for energy release. (1 mark)
- Example: Starch is hydrolyzed to glucose, which enters glycolysis. (1 mark)
- Both reactions are catalyzed by specific enzymes, ensuring efficient metabolism. (1 mark)
- Together, these processes balance energy storage and release in cells. (1 mark)