02.01 Biochemistry Principals
1. Basic Chemical Concepts
Atoms and Elements
- Atoms: The fundamental units of matter, consisting of protons, neutrons, and electrons.
- Elements: Pure substances composed of only one type of atom, each defined by its atomic number (number of protons).
Molecules and Compounds
- Molecules: Two or more atoms bonded together, representing the smallest unit of a chemical compound that retains its properties.
- Compounds: Substances formed when two or more different elements combine chemically in fixed proportions (e.g., water, CO₂).
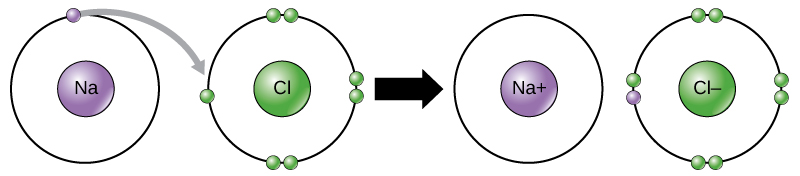
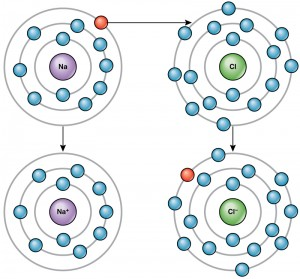
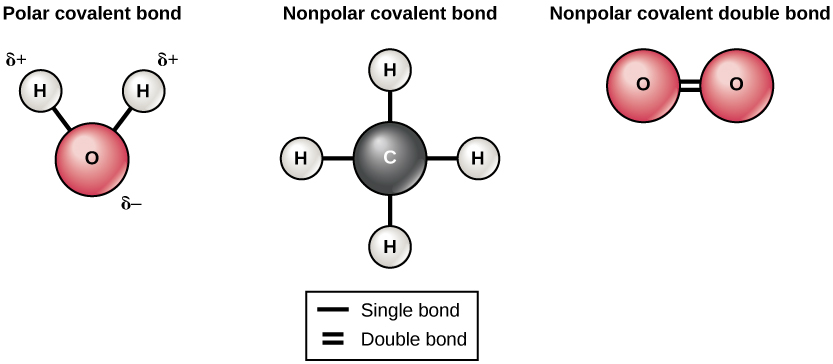
Chemical Bonds
- Ionic Bonds: Formed when electrons are transferred from one atom to another, resulting in oppositely charged ions that attract each other.
- Covalent Bonds: Involve the sharing of electron pairs between atoms, creating a strong bond.
- Hydrogen Bonds: Weak bonds between a hydrogen atom and a highly electronegative atom (such as oxygen or nitrogen) in another molecule.
- Van der Waals Forces: Weak, non-specific interactions caused by temporary dipoles in molecules.
2. Organic Chemistry
Role of Carbon
- Carbon’s Versatility: Carbon has four valency electrons, allowing it to form four covalent bonds with other atoms, including other carbon atoms. This property enables the formation of complex and diverse organic molecules.
- Catenation: The ability of carbon to form long chains and rings, providing a backbone for complex structures.
Hydrophobic and Hydrophilic Interactions
- Hydrophobic: Molecules or regions of molecules that repel water, typically nonpolar substances (e.g., lipid tails in phospholipids).
- Hydrophilic: Molecules or regions that attract water, typically polar or charged substances (e.g., hydroxyl groups in sugars).
Monomers and Polymers
- Monomers: Small, simple molecules that can join together to form larger, more complex molecules.
- Examples: Glucose (monomer of carbohydrates), amino acids (monomers of proteins), nucleotides (monomers of nucleic acids), fatty acids and glycerol (components of lipids).
- Polymers: Large, complex molecules made up of repeating monomer units.
- Examples:
- Carbohydrates: Polysaccharides like starch and cellulose.
- Proteins: Polymers of amino acids.
- Nucleic Acids: DNA and RNA, polymers of nucleotides.
- Lipids: While not always true polymers, they are composed of fatty acid chains and glycerol.
- Examples:
Functional Groups
- Hydroxyl (-OH): Found in alcohols and carbohydrates.
- Carbonyl (C=O): Present in ketones and aldehydes.
- Amino (-NH₂): Found in amino acids.
- Phosphate (-PO₄³⁻): Present in nucleotides and ATP.
- Carboxyl (-COOH): Found in fatty acids and amino acids.
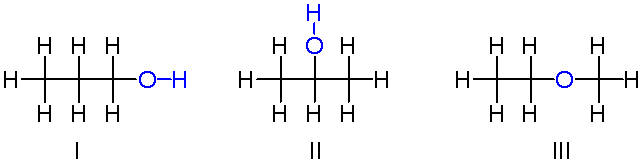
Isomerism
- Structural Isomers: Compounds with the same molecular formula but different bonding arrangements.
- Stereoisomers: Compounds with the same molecular formula and bonding but different spatial arrangements.
3. Metabolism
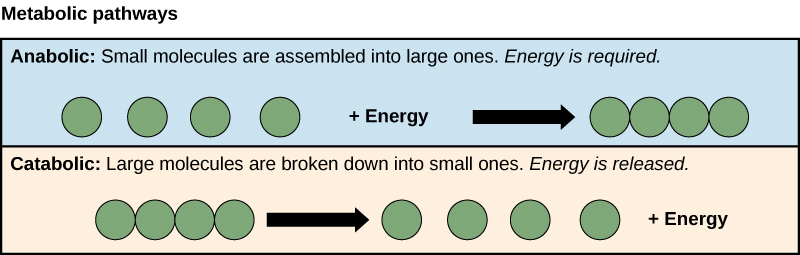
Catabolism and Anabolism
- Catabolism: Metabolic pathways that break down complex molecules into simpler ones, releasing energy.
- Examples: Glycolysis, the citric acid cycle, and fatty acid oxidation.
- Products: ATP, NADH, and other energy carriers.
- Anabolism: Metabolic pathways that build complex molecules from simpler ones, requiring energy.
- Examples: Protein synthesis, DNA replication, and gluconeogenesis.
- Products: Proteins, nucleic acids, and complex carbohydrates.
Enzymes
- Function: Biological catalysts that speed up chemical reactions by lowering the activation energy.
- Enzyme Kinetics: Study of the rates of enzyme-catalyzed reactions.
- Regulation: Enzyme activity can be controlled by inhibitors, activators, and feedback mechanisms.
4. Energy Transfer and Thermodynamics
ATP (Adenosine Triphosphate)
- Role: The primary energy carrier in cells.
- ATP Hydrolysis: The reaction ATP → ADP + Pi releases energy used for cellular processes.
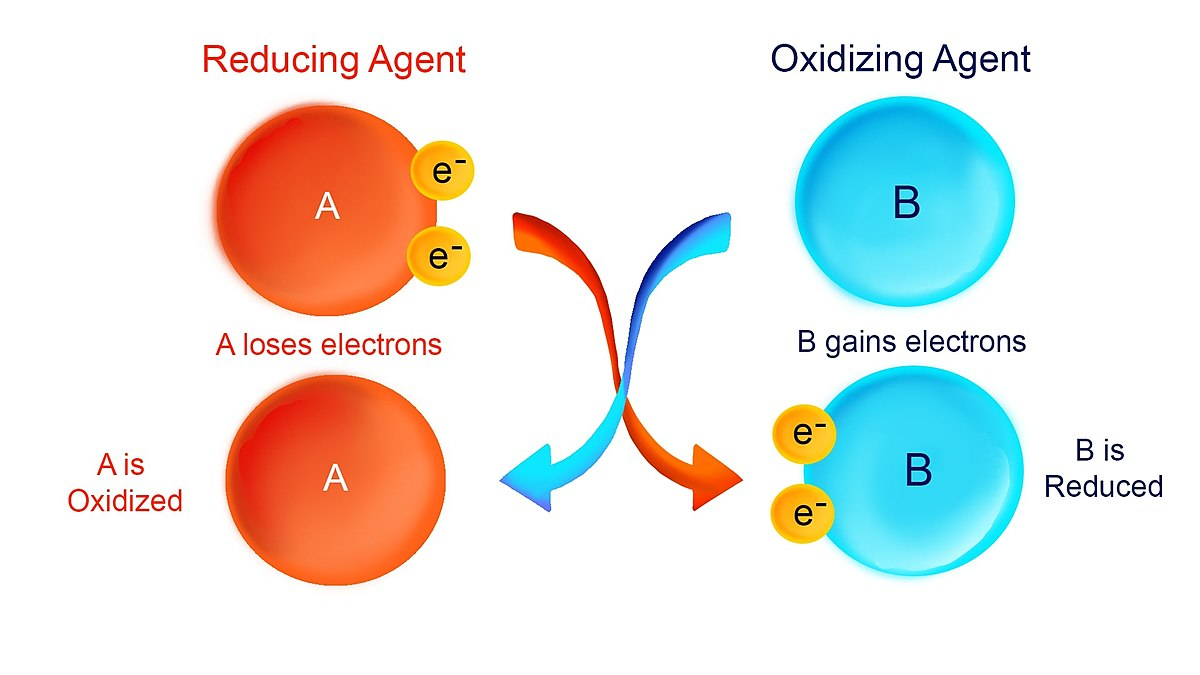
Redox Reactions
- Oxidation: Loss of electrons.
- Reduction: Gain of electrons.
- Electron Transport Chain: Series of redox reactions in cellular respiration that generate ATP.
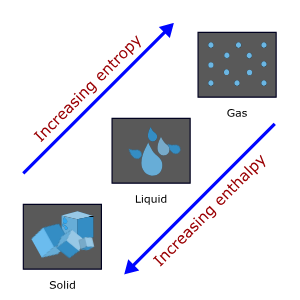
Thermodynamics
- Enthalpy (ΔH): Total heat content of a system.
- Entropy (ΔS): Measure of disorder or randomness in a system.
- Gibbs Free Energy (ΔG): Determines the spontaneity of a reaction (ΔG = ΔH – TΔS). Negative ΔG indicates a spontaneous reaction.
5. pH and Buffer Systems
pH Scale
- Range: 0-14, measuring the acidity or basicity of a solution.
- Acid: pH < 7
- Base: pH > 7
- Neutral: pH = 7
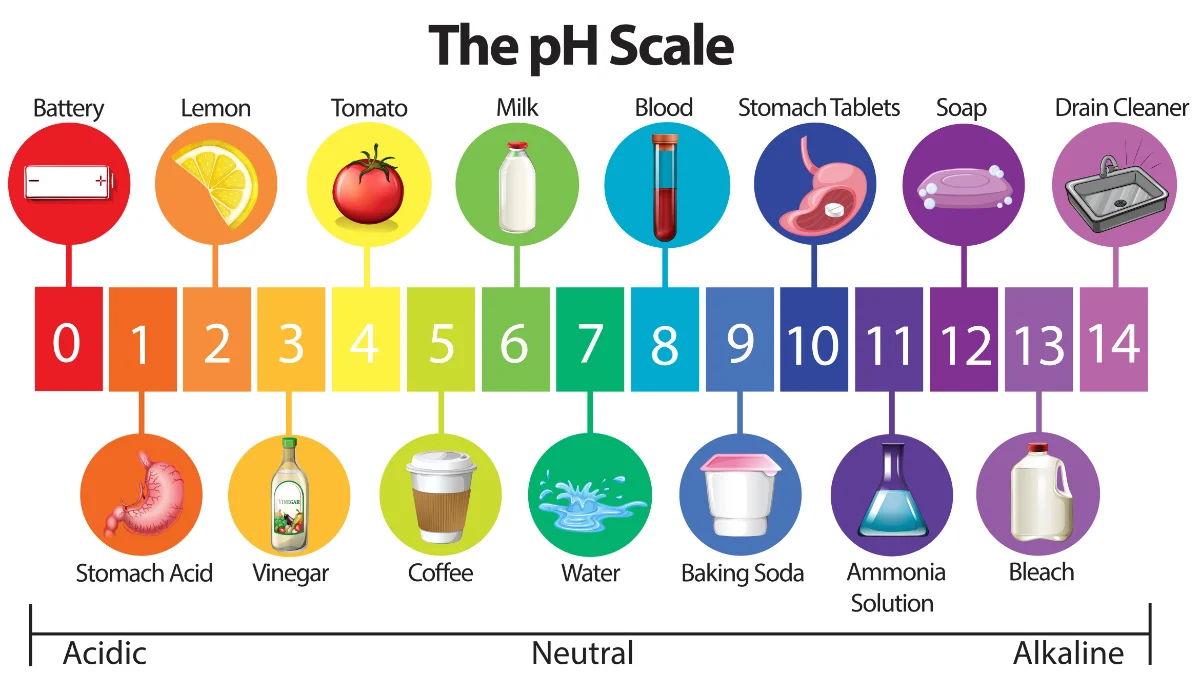
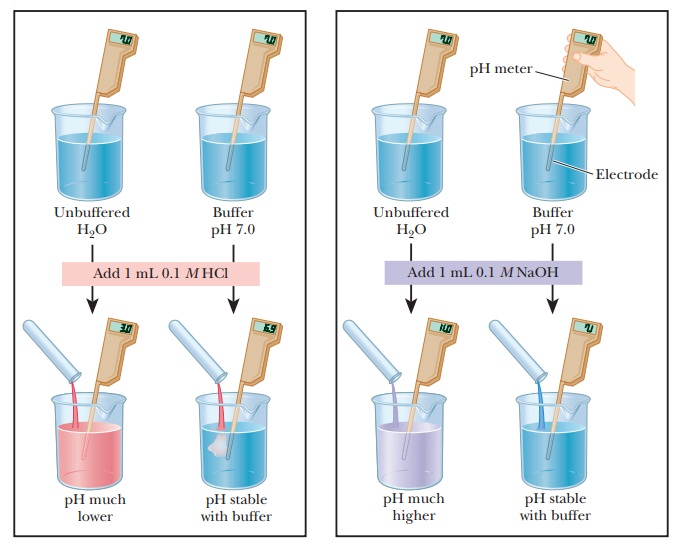
Buffers
- Function: Solutions that resist changes in pH by neutralizing added acids or bases.
- Biological Importance: Maintain the optimal pH for enzymatic activities and cellular functions.
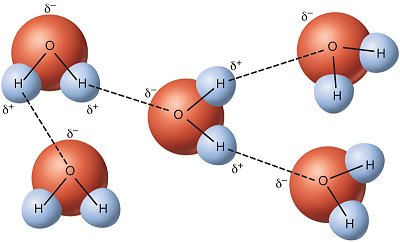
6. Properties of Water
Polarity
- Structure: Water molecules have a partial positive charge on hydrogen atoms and a partial negative charge on the oxygen atom.
- Hydrogen Bonding: Polar nature allows water to form hydrogen bonds, contributing to its unique properties.
Cohesion and Adhesion
- Cohesion: Attraction between water molecules, leading to surface tension.
- Adhesion: Attraction between water molecules and other substances, aiding in processes like capillary action.
High Specific Heat
- Definition: Ability of water to absorb or release large amounts of heat with minimal temperature change.
- Biological Significance: Helps regulate temperature in organisms and environments.
Solvent Properties
- Universal Solvent: Dissolves many substances, facilitating biochemical reactions and nutrient transport.
- Hydrophilic and Hydrophobic Interactions: Determines solubility and interactions of molecules in aqueous environments.
7. Additional Key Concepts
Chemical Equilibrium
- Definition: A state where the rate of the forward reaction equals the rate of the reverse reaction.
- Biological Relevance: Many biochemical reactions are reversible and exist in equilibrium.
- Le Chatelier’s Principle: If a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium moves to counteract the change.
- Examples in Biology: Binding of oxygen to hemoglobin, enzyme-substrate interactions.
Acid-Base Chemistry
- Importance: Critical for enzyme function, metabolic pathways, and maintaining cellular pH.
- Buffers: Systems that maintain acid-base balance in biological fluids.
- Buffer Systems in the Body: Bicarbonate buffer system, phosphate buffer system, protein buffers.
- pKa: The pH at which half of the buffer’s species are deprotonated, important for understanding buffer capacity.
Isotopes and Atomic Mass
- Mass Spectrometry: Technique used to determine the composition of isotopes in molecules, aiding in metabolic studies.
- Isotopes: Atoms of the same element with different numbers of neutrons.
- Stable vs. Radioactive Isotopes: Stability affects their roles in biological systems and tracing mechanisms.
- Atomic Mass: Weighted average mass of an element’s isotopes, important in biochemical calculations and tracing metabolic pathways.
Practise Questions
Question 1
Differentiate between an atom, molecule, and compound with examples. (5 marks)
Mark Scheme:
- Atom: The smallest unit of an element, consisting of protons, neutrons, and electrons. (1 mark)
Example: A hydrogen atom (H). (1 mark) - Molecule: Two or more atoms chemically bonded, representing the smallest unit of a compound retaining its properties. (1 mark)
Example: A water molecule (H₂O). (1 mark) - Compound: A substance formed when two or more different elements chemically combine in fixed proportions. (1 mark)
Example: Carbon dioxide (CO₂).
Question 2
Explain how covalent and ionic bonds differ in terms of electron behavior, providing examples. (6 marks)
Mark Scheme:
- Covalent Bonds: Formed by the sharing of electron pairs between atoms. (1 mark)
- Example: In H₂O, each hydrogen atom shares an electron with oxygen. (1 mark)
- Ionic Bonds: Formed by the transfer of electrons from one atom to another, creating oppositely charged ions. (1 mark)
- Example: In NaCl, sodium donates an electron to chlorine, forming Na⁺ and Cl⁻ ions. (1 mark)
- Covalent bonds are stronger, often forming stable molecules. (1 mark)
- Ionic bonds form between ions with opposite charges and are weaker in aqueous solutions. (1 mark)
Question 3
Describe the structure and function of monomers and polymers, with examples for carbohydrates and proteins. (6 marks)
Mark Scheme:
- Monomers are small, simple molecules that can join to form larger structures. (1 mark)
Example: Glucose (monomer of carbohydrates). (1 mark) - Polymers are large molecules made of repeating monomer units. (1 mark)
Example: Starch (polymer of glucose). (1 mark) - For proteins, the monomers are amino acids, linked by peptide bonds. (1 mark)
- Proteins are polymers made up of amino acids, functioning in structure (e.g., collagen) or enzymes (e.g., amylase). (1 mark)
Question 4
Explain the role of carbon in forming organic molecules. (5 marks)
Mark Scheme:
- Carbon’s four valence electrons allow it to form four covalent bonds with other atoms. (1 mark)
- This property enables the formation of long chains (catenation) and rings, providing structural diversity. (1 mark)
- Carbon can form bonds with other elements like hydrogen, oxygen, nitrogen, and itself. (1 mark)
- This versatility is key to the formation of complex molecules such as carbohydrates, lipids, proteins, and nucleic acids. (1 mark)
- Example: Carbon forms the backbone of glucose, a simple sugar essential for energy. (1 mark)
Question 5
What are functional groups, and how do they influence the properties of organic molecules? Provide two examples. (6 marks)
Mark Scheme:
- Functional groups are specific groups of atoms within molecules that determine their chemical properties. (1 mark)
- They influence reactivity, polarity, and interactions with other molecules. (1 mark)
- Example 1: The hydroxyl group (-OH) in alcohols increases polarity, making molecules hydrophilic. (1 mark)
- Example 2: The carboxyl group (-COOH) in fatty acids acts as an acid, donating H⁺ ions. (1 mark)
- Functional groups are crucial for enzyme-substrate interactions and metabolic reactions. (1 mark)
- Example: Amino (-NH₂) and carboxyl (-COOH) groups in amino acids are essential for forming peptide bonds. (1 mark)
Question 6
Compare catabolism and anabolism in terms of energy flow and examples. (5 marks)
Mark Scheme:
- Catabolism breaks down complex molecules into simpler ones, releasing energy. (1 mark)
Example: Glycolysis, where glucose is broken down to produce ATP. (1 mark) - Anabolism builds complex molecules from simpler ones, requiring energy input. (1 mark)
Example: Protein synthesis, where amino acids are joined to form proteins. (1 mark) - These processes are complementary, forming part of the metabolic cycle, with catabolism providing energy for anabolism. (1 mark)
Question 7
What is the role of ATP in cellular metabolism, and how is energy released? (5 marks)
Mark Scheme:
- ATP (Adenosine Triphosphate) is the primary energy carrier in cells. (1 mark)
- It stores energy in the high-energy phosphate bonds. (1 mark)
- Energy is released when ATP is hydrolyzed to ADP and Pi (inorganic phosphate). (1 mark)
- This energy is used for cellular processes like active transport, protein synthesis, and muscle contraction. (1 mark)
- ATP is regenerated in processes like cellular respiration, ensuring a continuous energy supply. (1 mark)
Question 8
Describe how hydrogen bonding contributes to water’s properties, including cohesion and high specific heat. (6 marks)
Mark Scheme:
- Hydrogen bonds form between the partial positive charge (δ+) of hydrogen in one water molecule and the partial negative charge (δ−) of oxygen in another. (1 mark)
- Cohesion: Hydrogen bonding holds water molecules together, creating surface tension. (1 mark)
- Example: Cohesion supports capillary action in plants, allowing water transport. (1 mark)
- High Specific Heat: Hydrogen bonds absorb significant energy, requiring more heat to raise water’s temperature. (1 mark)
- This property helps regulate temperature in organisms and environments. (1 mark)
- Hydrogen bonding also contributes to water’s ability to act as a universal solvent. (1 mark)
Question 9
Explain how buffers maintain pH in biological systems, providing an example. (5 marks)
Mark Scheme:
- Buffers resist changes in pH by neutralizing added acids or bases. (1 mark)
- They maintain a stable pH, critical for enzymatic activity and cellular functions. (1 mark)
- Example: The bicarbonate buffer system in blood maintains pH around 7.4. (1 mark)
- If H⁺ ions are added, bicarbonate (HCO₃⁻) binds them to form carbonic acid (H₂CO₃). (1 mark)
- If OH⁻ ions are added, carbonic acid releases H⁺ to neutralize the base, keeping pH stable. (1 mark)
Question 10
Summarize the significance of redox reactions in cellular respiration. (6 marks)
Mark Scheme:
- Redox reactions involve the transfer of electrons, with oxidation being the loss of electrons and reduction being the gain of electrons. (1 mark)
- In cellular respiration, glucose is oxidized, and oxygen is reduced, producing energy. (1 mark)
- The electron transport chain uses redox reactions to create a proton gradient across the mitochondrial membrane. (1 mark)
- This gradient drives ATP synthesis through oxidative phosphorylation. (1 mark)
- Redox reactions enable the efficient transfer of energy from glucose to ATP. (1 mark)
- Example: NAD⁺ is reduced to NADH, which donates electrons to the electron transport chain. (1 mark)