02.00. Chapter Summary
BioCast:
1. Elements, Compounds & Mixtures
- Classification: All substances are classified as elements, compounds, or mixtures.
- Element:
- Definition: Pure substances consisting of only one type of atom with the same number of protons.
- Example: Oxygen (O), Carbon (C).
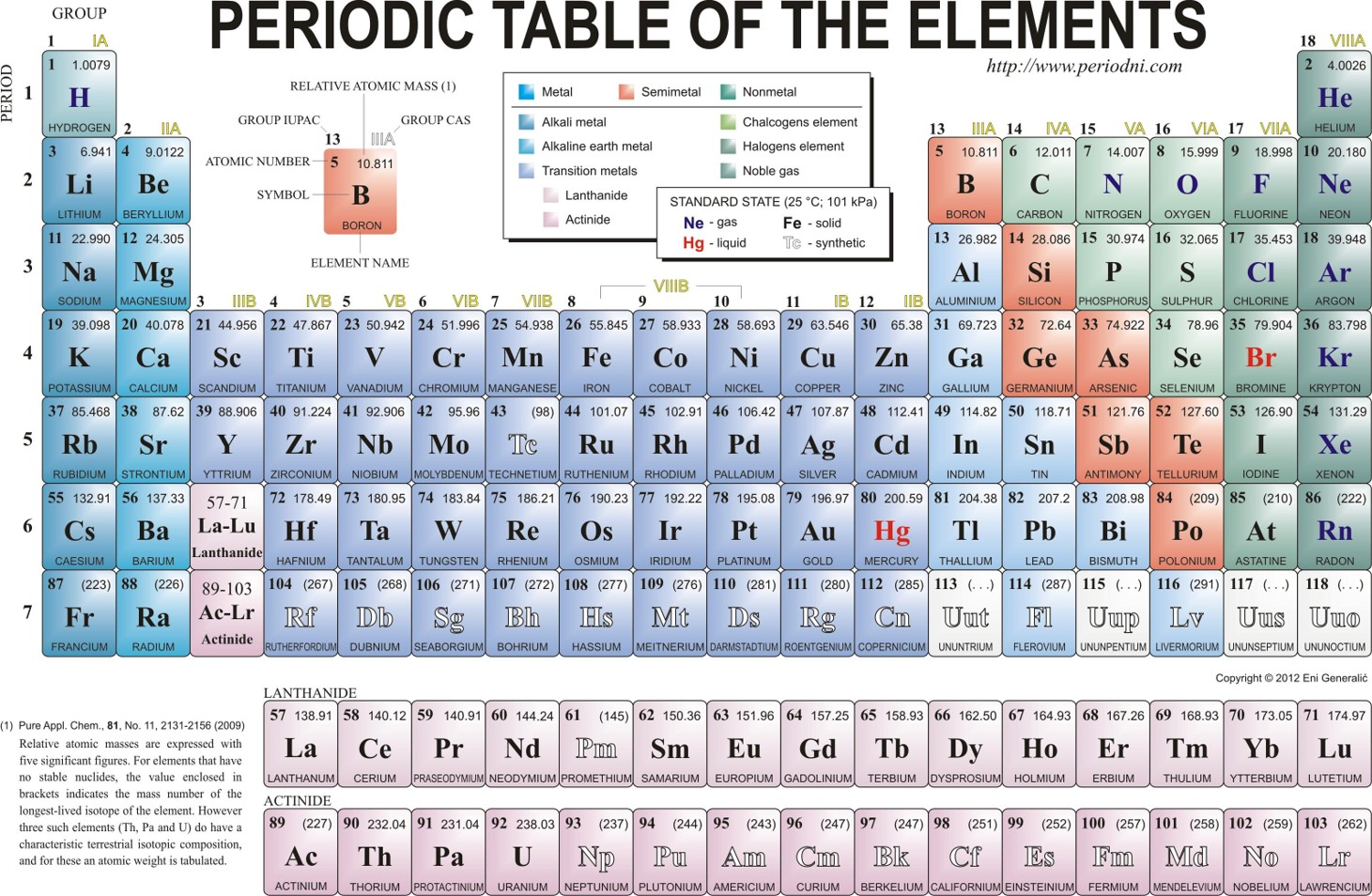
- Periodic Table: 118 elements are listed.
- Compound:
- Definition: Pure substances formed by chemically combining two or more elements in fixed proportions.
- Examples:
- Copper(II) sulfate (CuSO₄)
- Calcium carbonate (CaCO₃)
- Carbon dioxide (CO₂)
- Properties: Cannot be separated by physical means.
- Mixture:
- Definition: Combination of two or more substances (elements or compounds) physically mixed, not chemically bonded.
- Examples: Sand and water, oil and water, sulfur powder and iron filings.
- Properties: Can be separated by physical methods like filtration or evaporation.
- Diagrams:
- Particle Diagrams: Elements, compounds, and mixtures at the particle level.
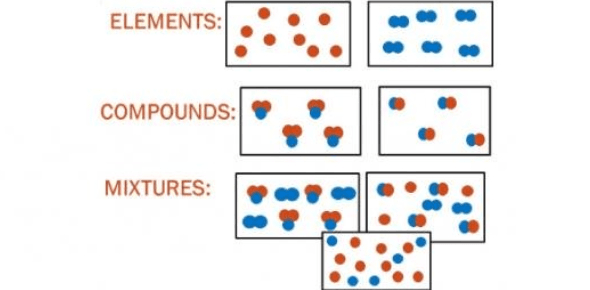
2. Atomic Structure and the Periodic Table
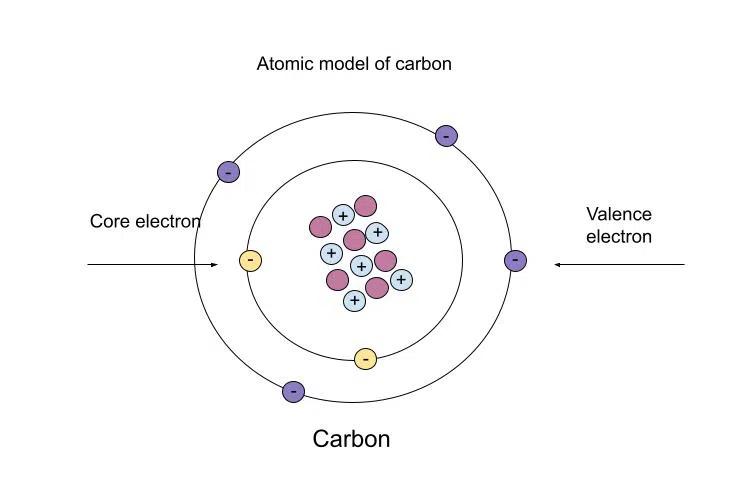
2.1 Structure of the Atom
- Basic Concepts:
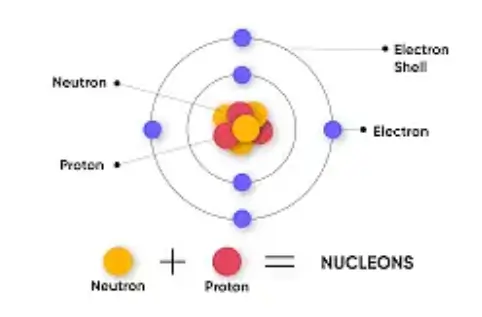
- Atom: Building block of matter, composed of protons, neutrons, and electrons.
- Subatomic Particles:
- Protons:
- Charge: +1
- Relative Mass: 1
- Neutrons:
- Charge: 0 (neutral)
- Relative Mass: 1
- Electrons:
- Charge: -1
- Relative Mass: ≈ 0 (negligible)
- Protons:
- Structure:
- Nucleus: Contains protons and neutrons.
- Electron Shells: Electrons orbit the nucleus in energy levels (shells).
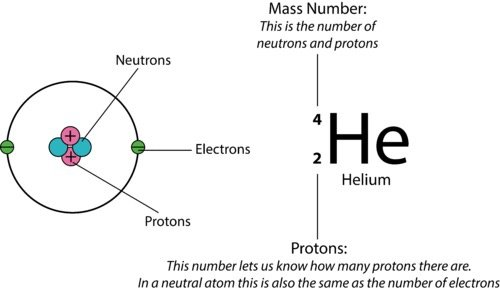
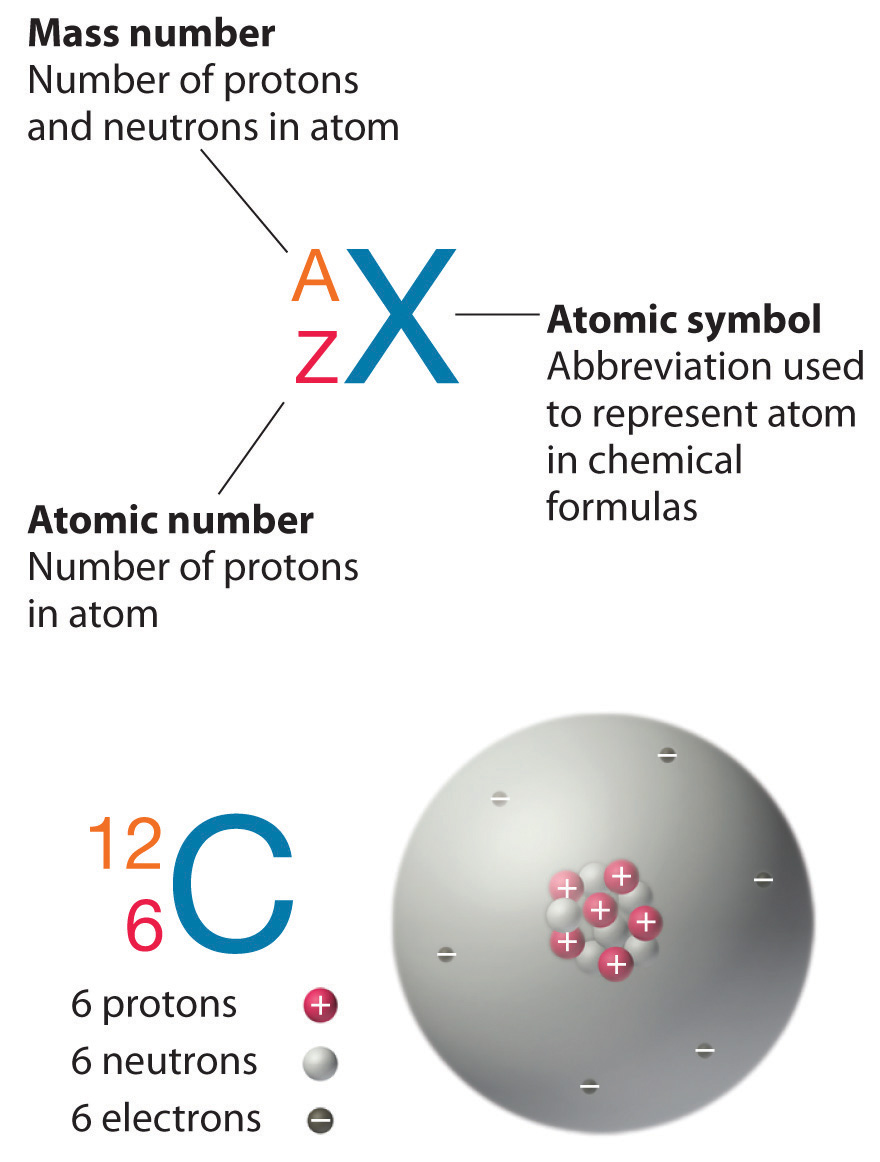
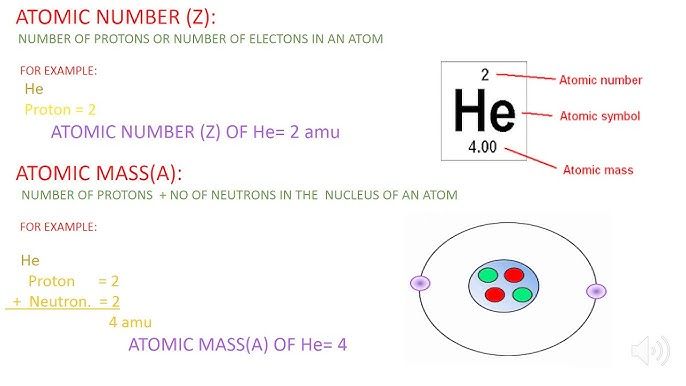
- Key Definitions:
- Atomic Number (Z): Number of protons in an atom; determines the element.
- Mass Number (A): Total number of protons and neutrons.
- Isotopes: Atoms of the same element with different numbers of neutrons.
- Diagram:
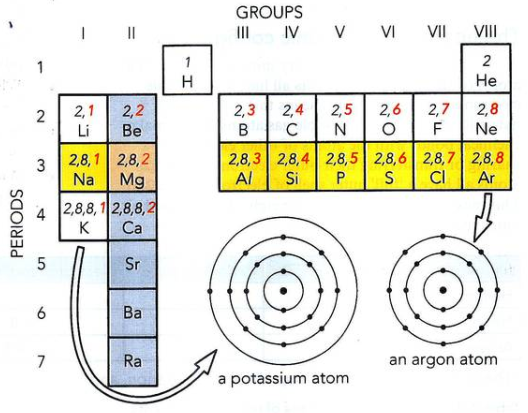
- Atomic Structure Diagrams: Protons, neutrons, and electrons within an atom. Notice that there are two ways that this information can be displayed.

- Periodic Table Layout: Illustrates elements with atomic number (Z) and relative atomic mass (Ar).
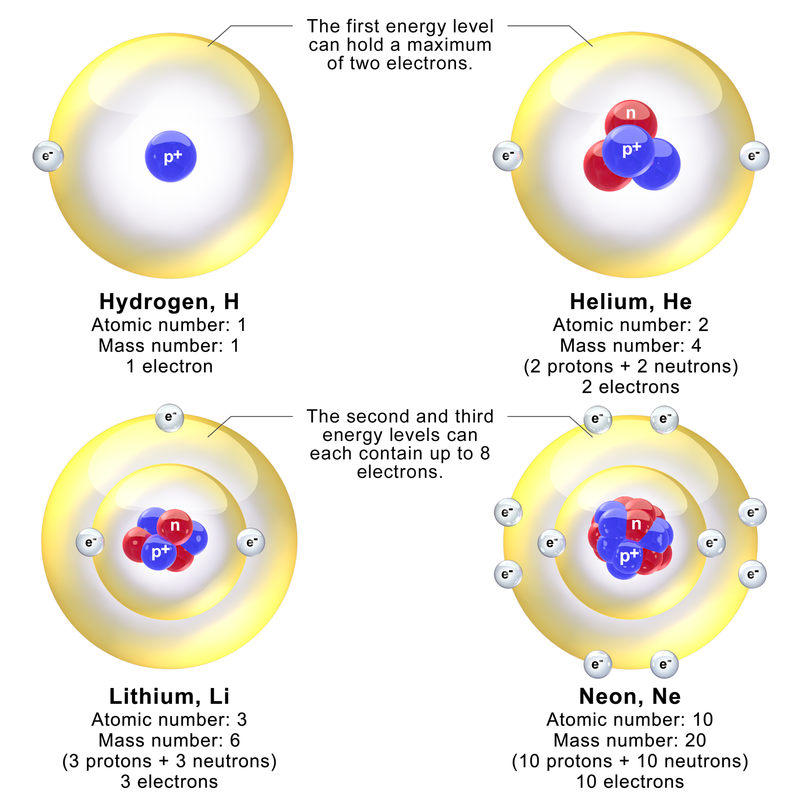
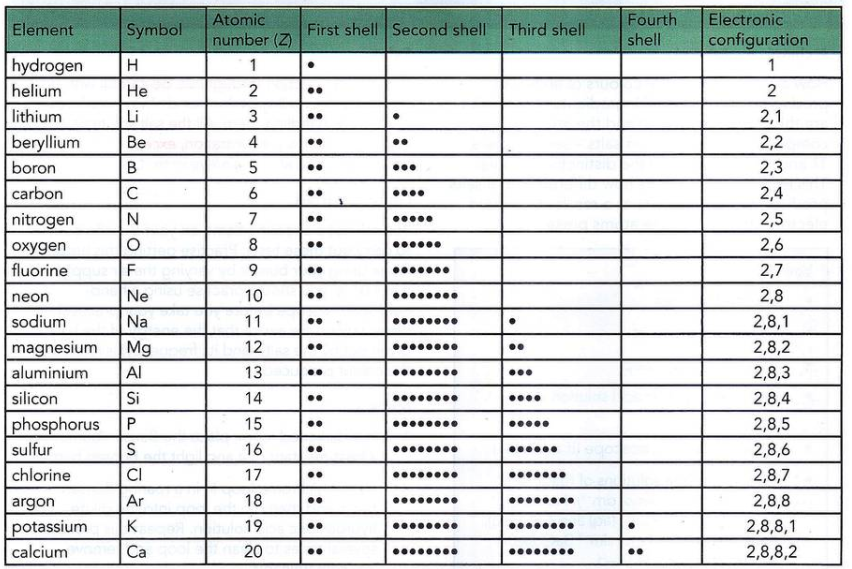
2.2 Electronic Configuration
- Concept:
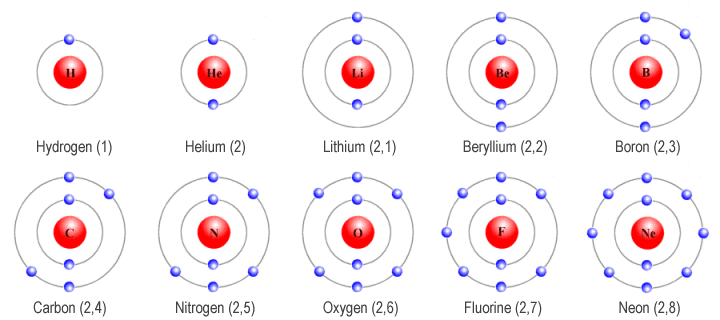
- Electronic Configuration: Arrangement of electrons in an atom’s shells.
- Notation: Numbers separated by commas indicate electrons in each shell (e.g., Carbon: 2,4).
- Rules:
- Electrons fill the lowest available energy levels first.
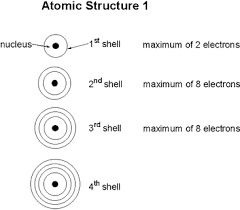
- Shell Capacities:
- 1st shell: 2 electrons
- 2nd shell: 8 electrons
- 3rd shell: 8 electrons (simplified for IGCSE)
- Periodic Table Relationship:
- Periods: Indicate the number of occupied shells.
- Groups: Indicate the number of valence (outer) electrons.
- Group VIII Noble Gases: Have a full outer shell.
- Groups I to VII: Number of outer shell electrons corresponds to the group number.
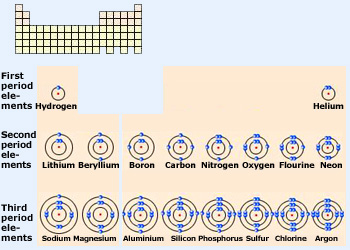
- Examples:
- Magnesium (Mg): 2,8,2
- Carbon (C): 2,4
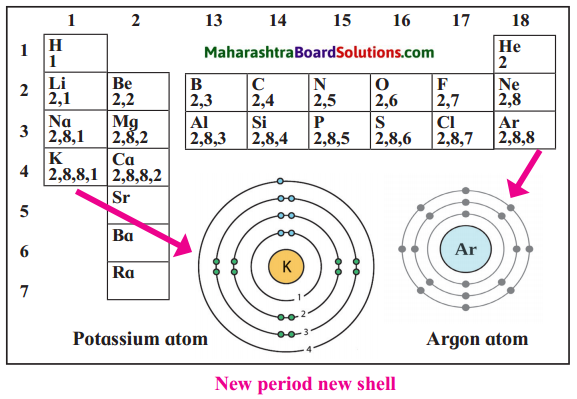
- Diagrams:
- Electronic Configuration Notation: Represent electron distribution in shells.
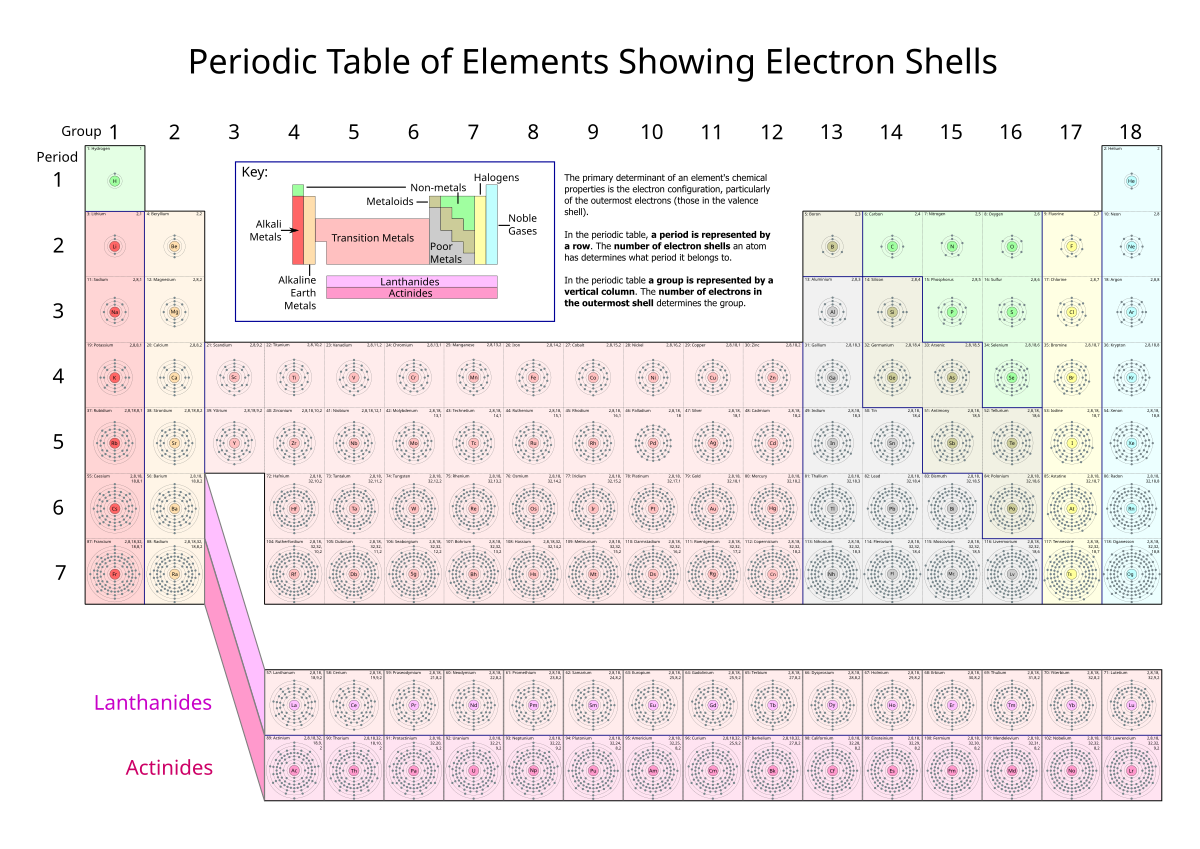
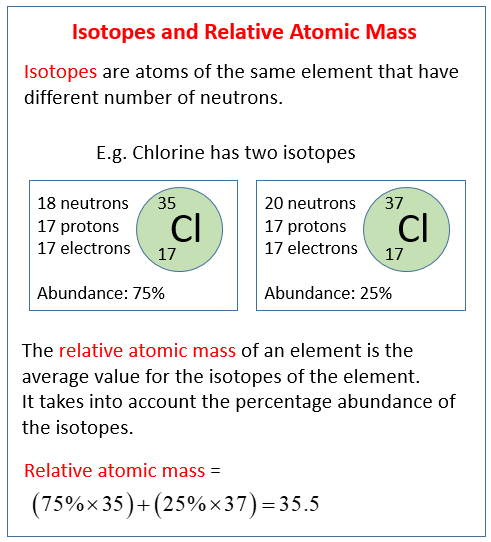
3. Isotopes and Relative Atomic Mass
3.1 Isotopes
- Definition: Different atoms of the same element that have the same number of protons but different numbers of neutrons.
- Symbol Representation:
- Standard Format: Element symbol with mass number as a superscript and atomic number as a subscript
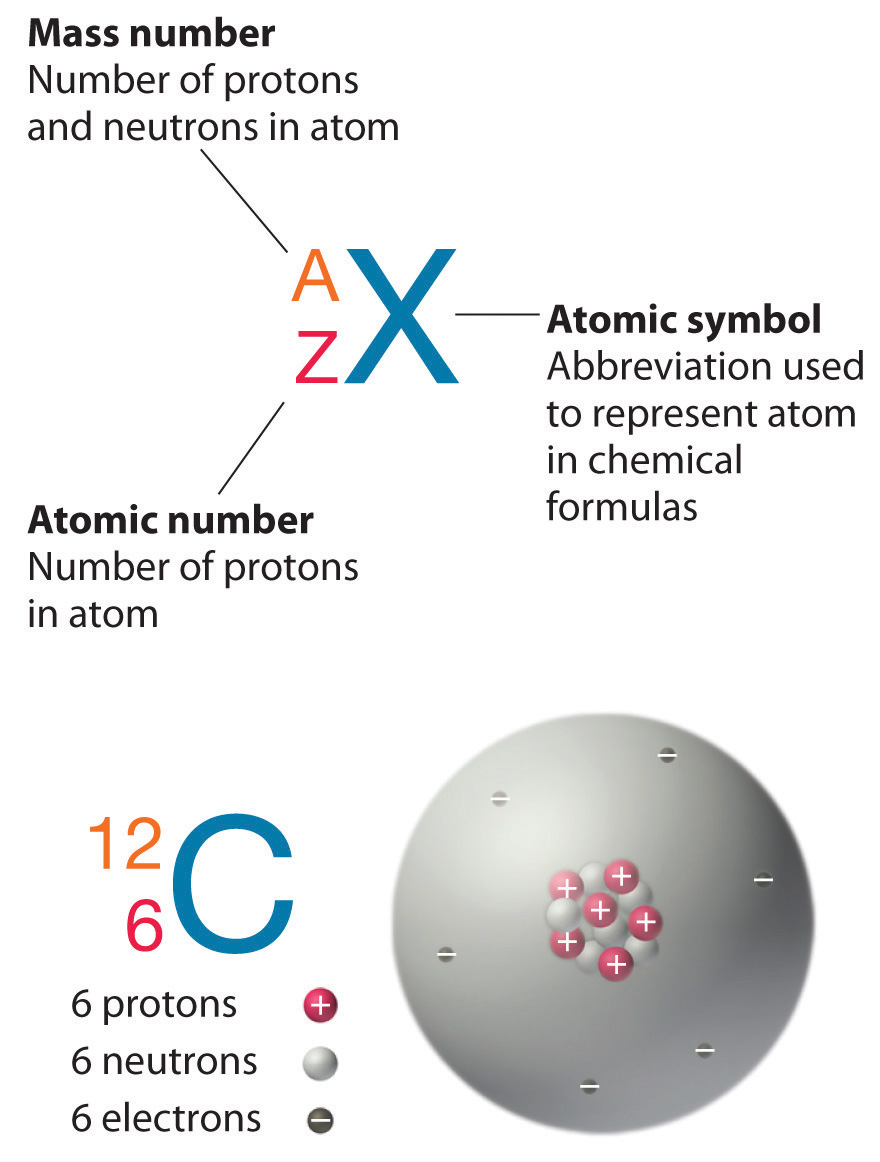
- Example:
- Carbon-12: ^12_6C
- Carbon-13: ^13_6C
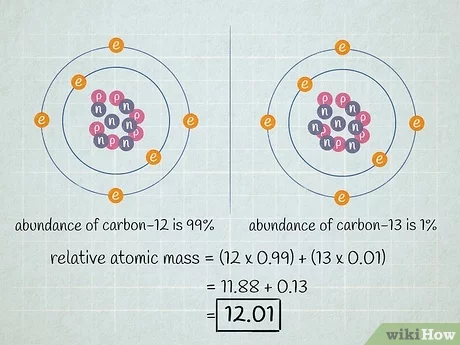
- Chemical Properties:
- Same Chemical Properties: Isotopes have identical chemical behavior due to identical electronic configurations.
- Different Physical Properties: Vary in mass, affecting properties like density and melting point.
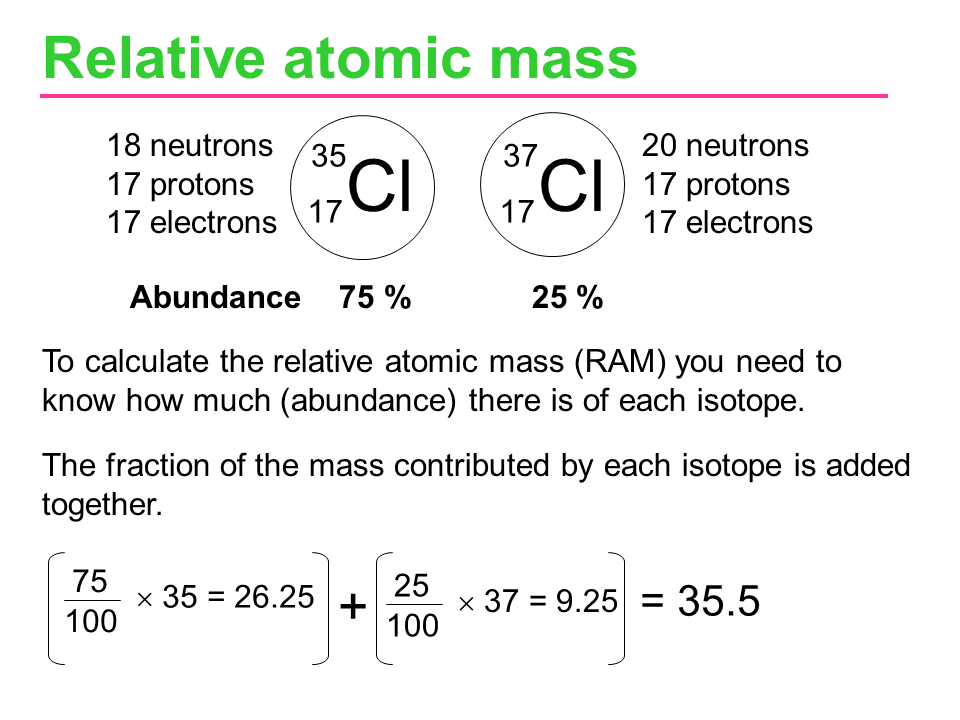
3.2 Relative Atomic Mass (Ar)
- Definition: Weighted average mass of an element’s isotopes compared to 1/12th the mass of carbon-12.
- Calculation:
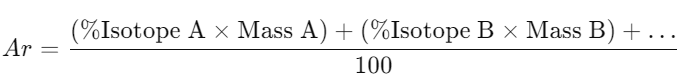
- Example:
- Rubidium:
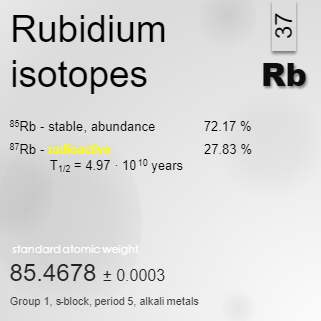
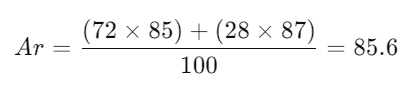
- Important Notes:
- Difference from Mass Number: Relative atomic mass accounts for all isotopes, whereas mass number refers to a specific isotope.
- No Units: It is a ratio and thus has no units.
4. Ions & Ionic Bonds
4.1 Formation of Ions
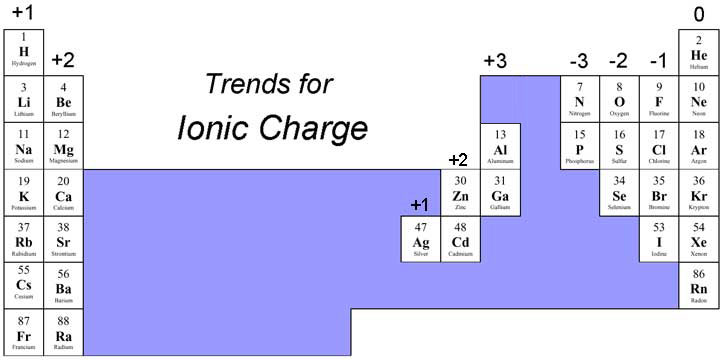
- Cations: Positive ions formed by losing electrons (typically metals).
- Example: Sodium ion (Na⁺)
- Anions: Negative ions formed by gaining electrons (typically non-metals).
- Example: Chloride ion (Cl⁻)
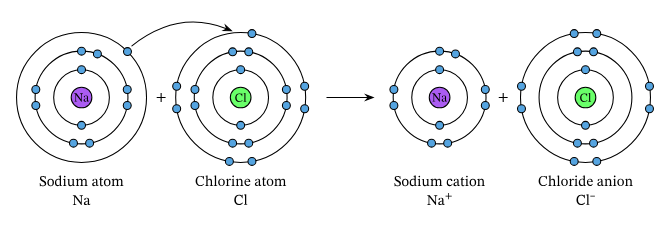
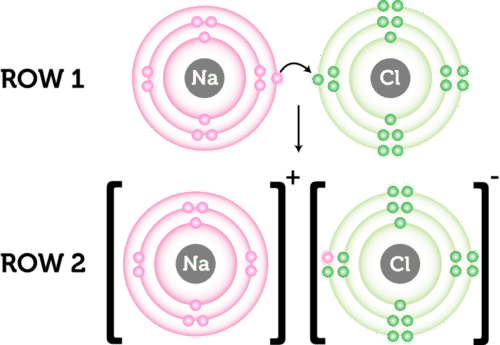
4.2 Ionic Bonding
- Definition: Strong electrostatic attraction between oppositely charged ions.
- Formation:
- Process: Transfer of electrons from metal to non-metal.
- Example:
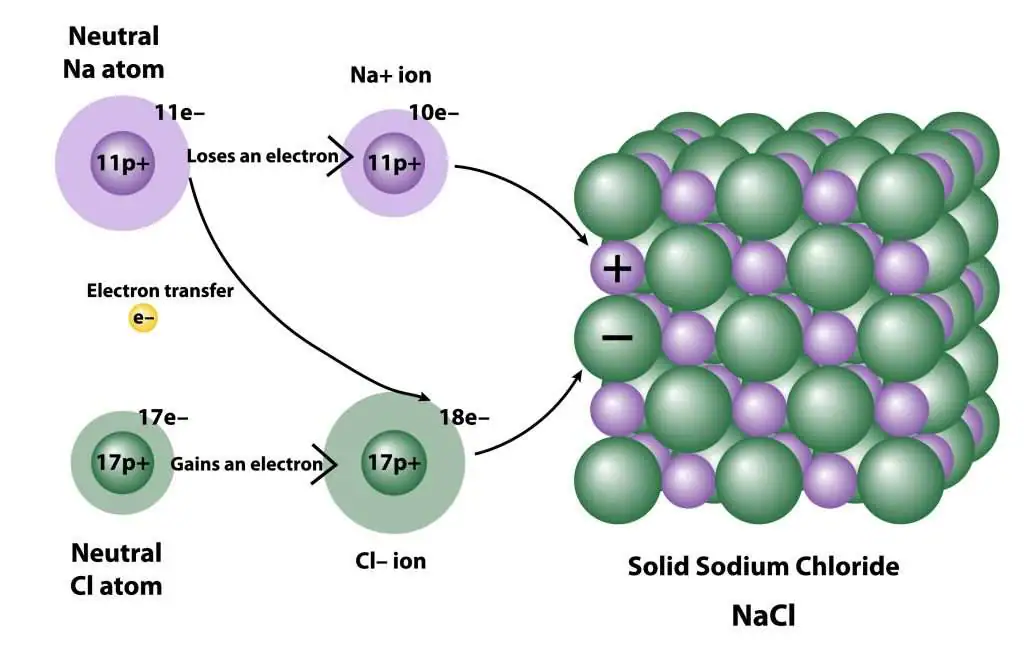
- Sodium Chloride (NaCl):
- Sodium (Na) loses one electron to form Na⁺.
- Chlorine (Cl) gains one electron to form Cl⁻.
- Na⁺ and Cl⁻ attract to form NaCl.
- Sodium Chloride (NaCl):
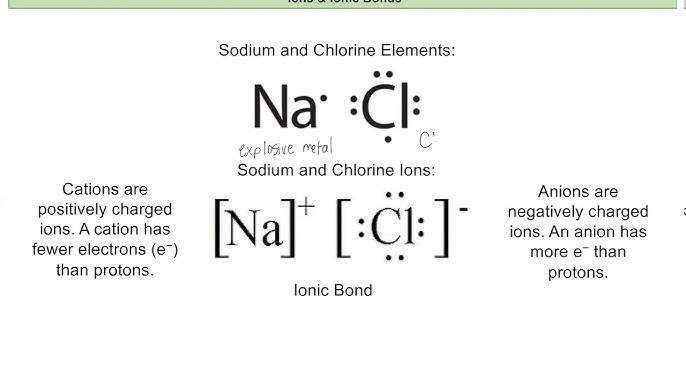
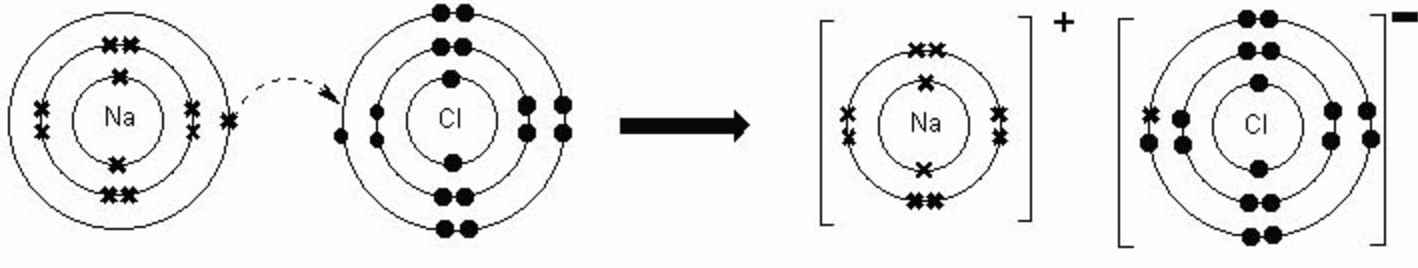
- Dot-and-Cross Diagrams:
- Ionic Bonds: Showing ions with charges enclosed in brackets.
- Example: NaCl
4.3 Properties of Ionic Compounds
- Physical State: Usually solid at room temperature.
- Melting & Boiling Points: High due to strong electrostatic forces in the lattice.
- Electrical Conductivity:
- Solid State: Poor conductors.
- Molten/Aqueous State: Good conductors due to free-moving ions.
- Structure:
- Giant Lattice Structure: Regular arrangement of alternating positive and negative ions.
- Example: NaCl lattice.
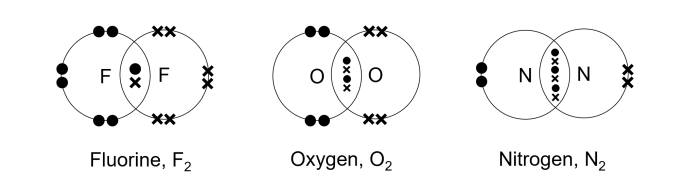
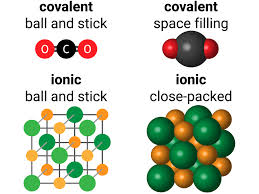
5. Simple Molecules and Covalent Bonds
5.1 Formation of Covalent Bonds
- Definition: Sharing of electron pairs between non-metal atoms to achieve noble gas electronic configurations.
- Molecules: Two or more atoms bonded covalently (e.g., H₂O, CO₂).
- Types of Covalent Bonds:
- Single Bond: Sharing one pair of electrons (e.g., H₂).
- Double Bond: Sharing two pairs of electrons (e.g., O₂).
- Triple Bond: Sharing three pairs of electrons (e.g., N₂).
5.2 Formation in Simple Molecules
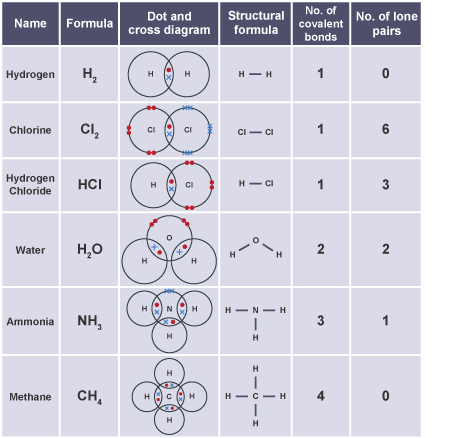
- Examples and Dot-and-Cross Diagrams:
- Hydrogen (H₂)
- Chlorine (Cl₂)
- Water (H₂O)
- Methane (CH₄)
- Ammonia (NH₃)
- Hydrogen Chloride (HCl)
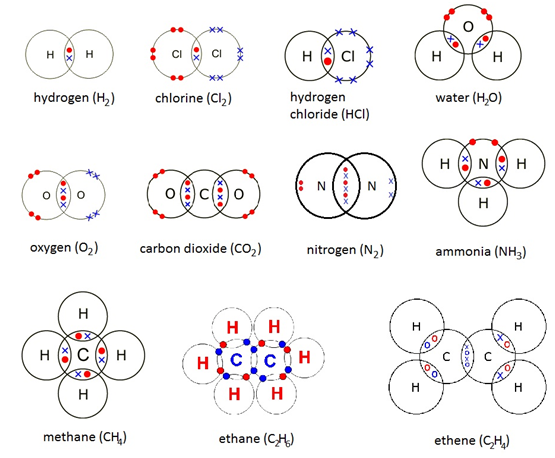
- Supplementary Examples:
- Methanol (CH₃OH)
- Ethene (C₂H₄)
- Carbon Dioxide (CO₂)
- Nitrogen (N₂)
5.3 Properties of Simple Molecular Compounds
- Physical State: Often gases or liquids at room temperature.
- Melting & Boiling Points: Generally low due to weak intermolecular forces.
- Electrical Conductivity: Poor conductors as they lack free ions or electrons.
- Examples: Water (H₂O), Methane (CH₄), Ammonia (NH₃), Hydrogen Chloride (HCl).
6. Giant Covalent Structures
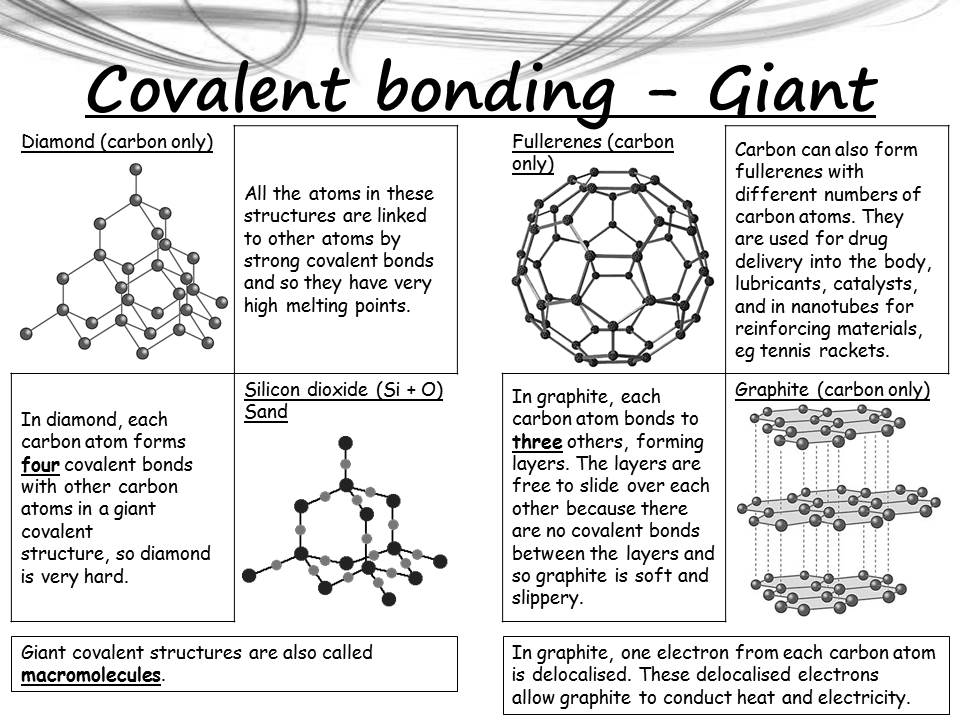
6.1 Graphite and Diamond
- Graphite:
- Structure: Layers of carbon atoms bonded in hexagons with delocalized electrons.
- Properties:
- Conducts electricity (due to delocalized electrons).
- Slippery (layers can slide over each other).
- High melting point.
- Uses: Pencils, lubricants, electrodes.
- Diamond:
- Structure: Each carbon atom bonded to four others in a tetrahedral lattice.
- Properties:
- Extremely hard.
- High melting point.
- Does not conduct electricity.
- Uses: Cutting tools, jewelry.
6.2 Silicon(IV) Oxide (SiO₂)
- Structure: Each silicon atom bonded to four oxygen atoms, forming a tetrahedral network similar to diamond.
- Properties:
- Very hard.
- High melting point.
- Insoluble in water.
- Uses: Sandpaper, furnace linings, glass production.
6.3 Similarities Between Diamond and SiO₂
- Properties:
- Both have giant covalent structures.
- Both are very hard and have high melting points.
- Both are insoluble in water.
- Neither conducts electricity.
7. Metallic Bonding
7.1 Structure of Metals
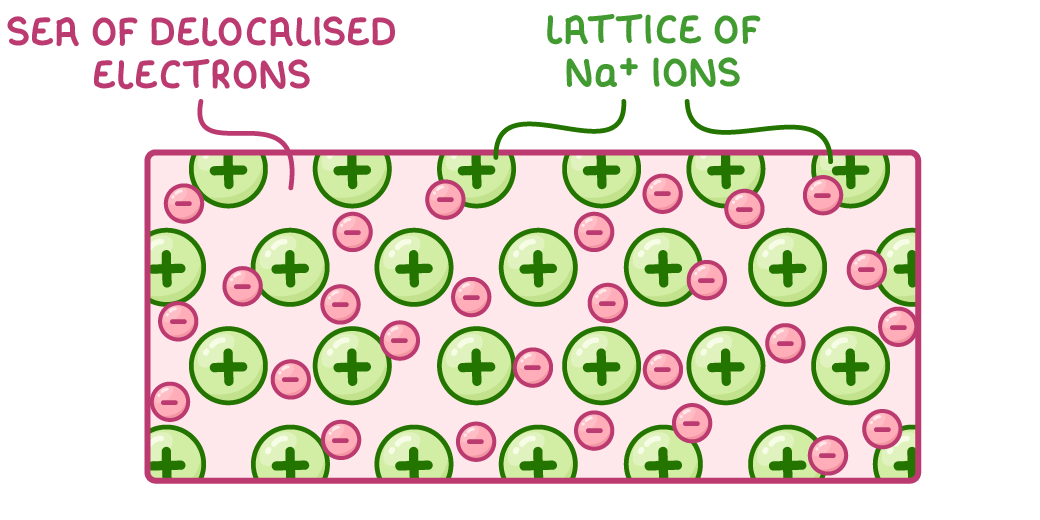
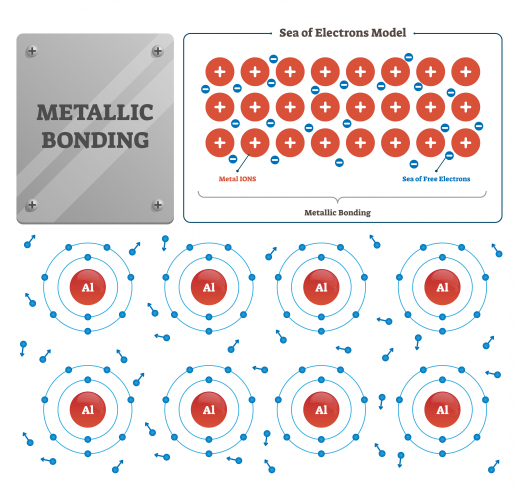
- Metal Lattice: Positive metal ions surrounded by a “sea of delocalized electrons.”
- Bonding: Electrostatic attraction between metal ions and free electrons.
7.2 Properties of Metals
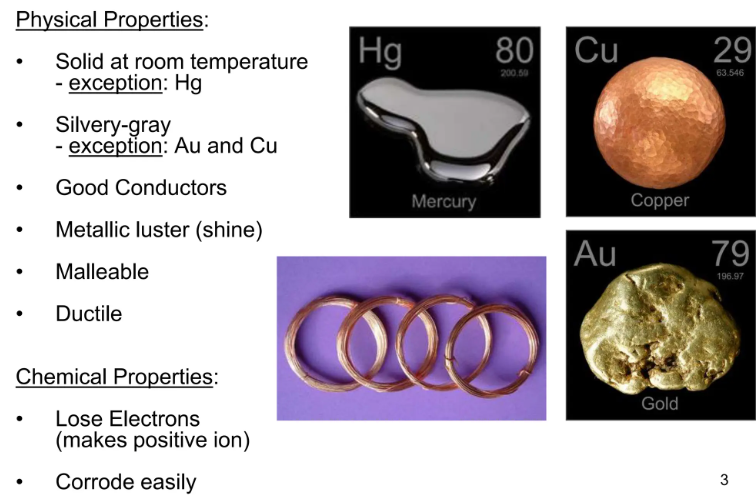
- Physical State: Mostly solid at room temperature (except mercury).
- Melting & Boiling Points: Generally high due to strong metallic bonds.
- Electrical & Thermal Conductivity: Excellent conductors (due to delocalized electrons).
- Malleability & Ductility:
- Malleability: Can be hammered into sheets.
- Ductility: Can be drawn into wires.
- Examples: Iron, Copper, Aluminum.
- Diagrams:
- Metallic Bonding Diagram: Shows metal ions in a lattice with delocalized electrons.
8. Electrical Conductivity in Compounds
- Ionic Compounds:
- Solid State: Poor conductors.
- Molten/Aqueous State: Good conductors (free-moving ions).
- Covalent Compounds:
- All States: Poor conductors (lack free ions or electrons).
- Metals:
- All States: Good conductors (delocalized electrons).
- Examples of Insulators:
- Covalent Compounds: Plastic, rubber, wood.
- Explanation: Lack free charge carriers.
9. Worked Examples
- Determining Atomic Particles:
- Example: Element X with atomic number 29 and mass number 63.
- Protons: 29
- Electrons: 29 (neutral atom)
- Neutrons: 63 – 29 = 34
- Example: Element X with atomic number 29 and mass number 63.
- Calculating Relative Atomic Mass:
- Rubidium Example:
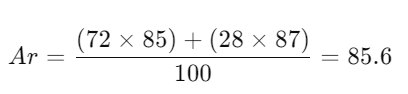
- Electronic Configuration:
- Magnesium (12 electrons): 2,8,2
- Ionic Bond Formation:
- Sodium Chloride (NaCl):
- Na: 2,8,1 → Na⁺: 2,8
- Cl: 2,8,7 → Cl⁻: 2,8,8
- Bond: Na⁺ and Cl⁻ form NaCl.
- Sodium Chloride (NaCl):
- Covalent Bond Formation:
- Water (H₂O):
- H: 1 electron each
- O: 6 electrons
- Configuration: H:O:H with shared electrons.
- Water (H₂O):
10. Exam Advice
- Electron Configurations:
- Master writing electronic configurations for the first 20 elements.
- Recognize patterns relating to periods and groups on the Periodic Table.
- Ion Formation:
- Use group numbers to determine ion charges:
- Group 1: +1
- Group 2: +2
- Group 6: -2
- Group 7: -1
- Group VIII: Noble gases (full outer shells).
- Use group numbers to determine ion charges:
- Bonding Diagrams:
- Ionic Bonds: Show ions with charges and use brackets.
- Covalent Bonds: Use dot-and-cross diagrams to represent shared electrons.
- Properties Correlation:
- Link bonding types to physical properties (e.g., lattice structures to high melting points in ionic compounds).
- Avoid Common Mistakes:
- Do not confuse mass number with relative atomic mass.
- Remember that graphite is a form of carbon, not the metal lead.
- Terminology:
- Valency Electrons: Electrons in the outer shell that determine bonding.
- Nucleons: Protons and neutrons collectively.
Quizzes
Quiz 1
1. Which of the following best defines a compound?
2. What particles are located in the nucleus of an atom?
3. How are mixtures typically separated?
4. What does the atomic number (Z) of an element represent?
5. What are isotopes?
6. How are cations formed?
7. What type of bond is formed by the transfer of electrons from one atom to another?
8. Which of the following is a property of ionic compounds?
9. How are metallic bonds characterized?
10. What is the relative atomic mass (Ar) of an element?
Quiz 2
1. What distinguishes a compound from a mixture?
2. Which subatomic particle determines the atomic number of an element?
3. How does the relative atomic mass (Ar) of an element differ from its mass number?
4. What is the process called when a substance changes directly from a solid to a gas?
5. Which of the following best describes the electronic configuration of magnesium (Mg)?
6. What type of bond is formed when chlorine gains an electron from sodium?
7. Which property is characteristic of metallic bonding?
8. What happens to the electrical conductivity of ionic compounds when they are molten?
9. Which of the following substances is an example of a giant covalent structure?
10. How does the electronic configuration relate to the position of an element in the Periodic Table?
Quiz 3
1. What is a mixture?
2. Which of the following is an example of a compound?
3. What is the charge of an anion?
4. How are covalent bonds formed?
5. What property distinguishes a metal from a non-metal?
6. What is the electronic configuration of carbon (C)?
7. What is the process called when an atom gains electrons?
8. Which of the following is a property of ionic compounds?
9. What is an example of a giant covalent structure?
10. How does the electronic configuration of an element influence its position in the Periodic Table?
Quiz 4
1. What defines an element?
2. Which of the following is a characteristic of compounds?
3. How can mixtures be separated?
4. What particles are found in the nucleus of an atom?
5. What does the atomic number (Z) represent?
6. What are isotopes?
7. How are cations formed?
8. What type of bond is formed when chlorine gains an electron from sodium?
9. Which property is characteristic of metallic bonding?
10. How does the electronic configuration of an element influence its position in the Periodic Table?
Practice Questions 1
Practice Questions 2