9.02 Haber Process and Contact Process
1. Overview of Industrial Processes
a. Importance:
- Haber Process: Critical for the large-scale synthesis of ammonia, which is essential for fertilizers and explosives.
- Contact Process: Fundamental for the industrial production of sulfuric acid, a key chemical in various industries including fertilizers, chemicals, and manufacturing.
b. Historical Context:
- Fritz Haber: Developed the Haber process in the early 20th century, revolutionizing agriculture and industry.
- Industrial Significance: Enabled the production of ammonia and sulfuric acid on an unprecedented scale, supporting population growth and industrial advancements.
2. Haber Process
a. Chemical Reaction:
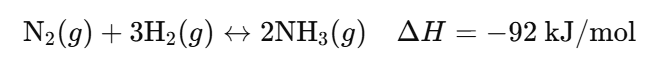
- Forward Reaction: Nitrogen and hydrogen gases combine to form ammonia (exothermic).
- Reverse Reaction: Ammonia decomposes back into nitrogen and hydrogen (endothermic).
b. Purpose:
- Nitrogen Fixation: Converts inert nitrogen gas (N₂) from the air into ammonia (NH₃), which can be used to produce fertilizers.
- Agricultural Importance: Ammonia is a precursor to nitrogenous fertilizers, essential for plant growth.
c. Conditions for the Haber Process:
| Condition | Details |
|---|---|
| Temperature | 450°C (Compromise Temperature) – Lowering favors ammonia production but slows the reaction. – Raising increases reaction rate but decreases ammonia yield. |
| Pressure | 20000 kPa (200 atmospheres) – Higher pressure favors ammonia production by shifting equilibrium to the right (fewer gas molecules). |
| Catalyst | Finely Divided Iron – Increases the rate of both forward and reverse reactions without altering equilibrium concentrations. |
| Reactant Ratio | Nitrogen to Hydrogen = 1:3 – Ensures optimal conditions for ammonia synthesis. |
d. Industrial Process Flow:
- Raw Materials:
- Nitrogen (N₂): Obtained from air via fractional distillation or chemical reactions.
- Hydrogen (H₂): Produced by steam reforming of natural gas (methane) or catalytic cracking of hydrocarbons.
- Production Steps:
- Steam Reforming:
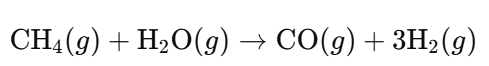
- Water-Gas Shift Reaction:
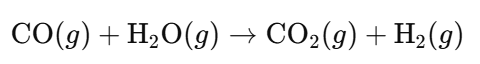
- Separation: Remove carbon dioxide to obtain pure hydrogen.
- Catalyst Use: Finely divided iron catalysts facilitate the ammonia synthesis.
- Ammonia Synthesis:
- Reaction Chamber: Gases are mixed in a 1:3 ratio, compressed to 20000 kPa, and heated to 450°C.
- Catalyst Beds: Gases pass over iron catalyst beds to facilitate reaction.
- Equilibrium Management: Remove ammonia as it forms to drive the reaction forward.
- Recycling: Unreacted nitrogen and hydrogen gases are recycled back into the reactor to maximize yield.
e. Yield Optimization:
- Pressure Increase: Shifts equilibrium to the right, increasing ammonia yield.
- Temperature Adjustment: Balance between higher yield (lower temperature) and acceptable reaction rate (higher temperature).
- Ammonia Removal: Continuously removing ammonia shifts equilibrium to produce more ammonia.
- Catalyst Efficiency: Enhances reaction rate, allowing equilibrium to be reached faster without changing the yield.
f. Practical Application:
- Industrial Plants: Modern Haber plants operate at around 450°C and 20000 kPa with iron catalysts.
- Yield: Approximately 15% ammonia is produced per pass; with recycling, yields can reach up to 98%.
g. Uses of Ammonia:
| Property | Ammonia (NH₃) |
|---|---|
| Appearance | Colorless gas with a pungent smell. |
| Solubility | Highly soluble in water, forming an alkaline solution. |
| Density | Denser than air. |
| Uses | Fertilizers (e.g., ammonium nitrate), explosives, cleaning agents, refrigerants. |
3. Contact Process
a. Chemical Reaction:
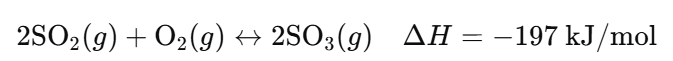
- Forward Reaction: Sulfur dioxide and oxygen gases combine to form sulfur trioxide (exothermic).
- Reverse Reaction: Sulfur trioxide decomposes back into sulfur dioxide and oxygen (endothermic).
b. Purpose:
- Sulfuric Acid Production: Converts sulfur dioxide (SO₂) into sulfur trioxide (SO₃), which is then used to produce sulfuric acid (H₂SO₄), a vital industrial chemical.
c. Conditions for the Contact Process:
| Condition | Details |
|---|---|
| Temperature | 450°C (Compromise Temperature) – Lower temperatures favor SO₃ production but slow the reaction. – Higher temperatures increase reaction rate but decrease yield. |
| Pressure | 200 kPa – Higher pressure favors SO₃ production by shifting equilibrium to the right (fewer gas molecules). |
| Catalyst | Vanadium(V) Oxide (V₂O₅) – Enhances the rate of both forward and reverse reactions without affecting equilibrium concentrations. |
d. Industrial Process Flow:
- Raw Materials:
- Sulfur (S): Mined or obtained from desulfurization of fossil fuels.
- Oxygen (O₂): Extracted from air via fractional distillation.
- Production Steps:
- Sulfur Combustion:
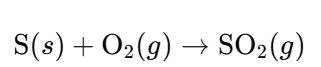
- Sulfur Dioxide Purification: Remove impurities to obtain pure SO₂.
- Sulfur Trioxide Synthesis:
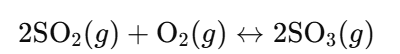
- Catalyst Use: V₂O₅ increases reaction rate.
- Sulfuric Acid Production:
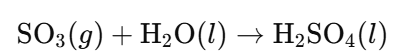
- Process: SO₃ is dissolved in existing H₂SO₄ to prevent formation of acid mist.
- Storage: Concentrated sulfuric acid (98% H₂SO₄) is stored and then diluted as needed.
e. Yield Optimization:
- Pressure Increase: Shifts equilibrium to the right, increasing SO₃ production.
- Temperature Adjustment: Balance between higher yield (lower temperature) and acceptable reaction rate (higher temperature).
- Catalyst Efficiency: Vanadium(V) oxide facilitates faster attainment of equilibrium.
f. Practical Application:
- Industrial Plants: Operate at around 450°C and 200 kPa with V₂O₅ catalysts.
- Yield: Approximately 98% conversion of SO₂ to SO₃ under optimal conditions.
g. Uses of Sulfuric Acid:
| Property | Sulfuric Acid (H₂SO₄) |
|---|---|
| Appearance | Colourless to slightly yellow liquid. |
| Properties | Highly corrosive, strong dehydrating agent, excellent solvent. |
| Uses | Fertilizers (e.g., ammonium sulfate), battery acid, mineral processing, chemical synthesis, detergents, paints, and pigments. |
4. Comparison of Haber and Contact Processes
| Feature | Haber Process | Contact Process |
|---|---|---|
| Purpose | Synthesis of ammonia (NH₃) | Production of sulfur trioxide (SO₃) for sulfuric acid (H₂SO₄) |
| Chemical Reaction | N₂(g) + 3H₂(g) ↔ 2NH₃(g) ΔH = -92 kJ/mol | 2SO₂(g) + O₂(g) ↔ 2SO₃(g) ΔH = -197 kJ/mol |
| Conditions | – Temperature: 450°C – Pressure: 20000 kPa – Catalyst: Iron | – Temperature: 450°C – Pressure: 200 kPa – Catalyst: V₂O₅ |
| Key Factors for Yield | – High pressure – Moderate temperature – Continuous removal of ammonia | – High pressure – Moderate temperature – Use of effective catalyst |
| Uses of Products | Fertilizers, explosives, cleaning agents | Sulfuric acid for fertilizers, chemicals, manufacturing |
| Industrial Scale | Over 150 million tonnes/year | Extensive production for global sulfuric acid supply |
5. Application of Le Chatelier’s Principle
a. Haber Process:
- Reaction: N₂(g) + 3H₂(g) ↔ 2NH₃(g) ΔH = -92 kJ/mol
- Exothermic Forward Reaction:
- Increasing Pressure: Shifts equilibrium to the right, increasing ammonia yield.
- Decreasing Pressure: Shifts equilibrium to the left, decreasing ammonia yield.
- Increasing Temperature: Shifts equilibrium to the left (reverse reaction), decreasing ammonia yield.
- Decreasing Temperature: Shifts equilibrium to the right (forward reaction), increasing ammonia yield.
- Removing Ammonia: Shifts equilibrium to the right, increasing ammonia production.
- Adding Nitrogen or Hydrogen: Shifts equilibrium to the right, increasing ammonia production.
- Adding a Catalyst: Speeds up the attainment of equilibrium without changing the position.
b. Contact Process:
- Reaction: 2SO₂(g) + O₂(g) ↔ 2SO₃(g) ΔH = -197 kJ/mol
- Exothermic Forward Reaction:
- Increasing Pressure: Shifts equilibrium to the right, increasing sulfur trioxide yield.
- Decreasing Pressure: Shifts equilibrium to the left, decreasing sulfur trioxide yield.
- Increasing Temperature: Shifts equilibrium to the left (reverse reaction), decreasing sulfur trioxide yield.
- Decreasing Temperature: Shifts equilibrium to the right (forward reaction), increasing sulfur trioxide yield.
- Adding Sulfur Dioxide or Oxygen: Shifts equilibrium to the right, increasing sulfur trioxide production.
- Removing Sulfur Trioxide: Shifts equilibrium to the right, increasing sulfur trioxide production.
- Adding a Catalyst: Speeds up the attainment of equilibrium without changing the position.
6. Key Terminology
- Haber Process: Industrial method for synthesizing ammonia from nitrogen and hydrogen.
- Contact Process: Industrial method for producing sulfur trioxide from sulfur dioxide and oxygen.
- Equilibrium: A state where the rate of the forward reaction equals the rate of the reverse reaction.
- Dynamic Equilibrium: Continuous forward and reverse reactions with no net change in concentrations.
- Le Chatelier’s Principle: Predicts the direction of shift in equilibrium when a change in conditions is applied.
- Exothermic Reaction: Releases heat energy.
- Endothermic Reaction: Absorbs heat energy.
- Catalyst: A substance that increases the rate of a chemical reaction without being consumed.
- Compromise Temperature: A temperature that provides a balance between reaction rate and yield.
- Fritz Haber: German chemist who developed the Haber process.
- Vanadium(V) Oxide (V₂O₅): Catalyst used in the Contact process.
- Iron Catalyst: Finely divided iron used in the Haber process.
Examples:
Question 1:
Oxygen will react with both nitrogen (N₂) and nitrogen(II) oxide (NO) in reversible reactions.
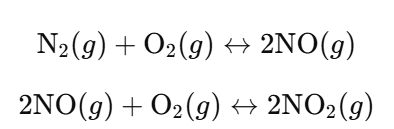
a. Describe how an increase in pressure will affect the position of equilibrium of:
- i. The reaction between nitrogen and oxygen.
- ii. The reaction between oxygen and nitrogen(II) oxide.
Answer:
- i. Reaction:
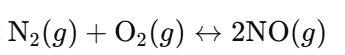
- Total Gas Molecules:
- Reactants: 1 N₂ + 1 O₂ = 2 molecules.
- Products: 2 NO = 2 molecules.
- Effect of Increasing Pressure: No shift in equilibrium (same number of gas molecules on both sides).
- ii. Reaction:
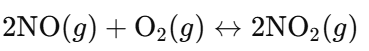
- Total Gas Molecules:
- Reactants: 2 NO + 1 O₂ = 3 molecules.
- Products: 2 NO₂ = 2 molecules.
- Effect of Increasing Pressure: Shifts equilibrium to the right (fewer gas molecules), increasing NO₂ production.
Question 2:
The availability of raw materials is essential for an industrial process.
a. How is hydrogen obtained for use in the Haber process?
Answer:
- Steam Reforming of Natural Gas (Methane):
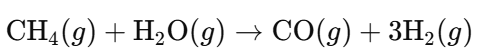
- Catalytic Cracking of Hydrocarbons (e.g., Ethane):
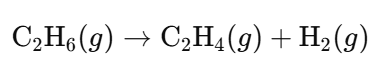
- Purification: Remove carbon dioxide and other impurities to obtain pure hydrogen for ammonia synthesis.
b. Describe two sources of sulfur dioxide for the Contact process.
Answer:
- Burning Sulfur:
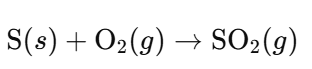
- Roasting Sulfide Ores (e.g., Zinc Blende, ZnS):
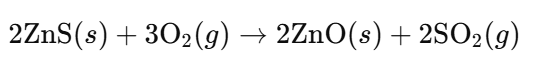
- Explanation: Sulfide ores are heated in the presence of oxygen to produce sulfur dioxide and metal oxide.
c. What is the common source of the nitrogen and oxygen required for the Haber and Contact processes?
Answer:
- Air: The primary source of both nitrogen (N₂) and oxygen (O₂). Air is separated into its components, typically through fractional distillation, to provide pure nitrogen and oxygen gases required for the Haber and Contact processes.
Question 3:
a. What are the conditions used for the Haber process?
Answer:
- Temperature: 450°C (compromise temperature)
- Pressure: 20000 kPa (200 atmospheres)
- Catalyst: Finely divided iron
- Reactant Ratio: Nitrogen (N₂) to Hydrogen (H₂) in a 1:3 ratio
- Ammonia Removal: Continuously remove ammonia from the reaction mixture to shift equilibrium towards more ammonia production
b. Will increasing the pressure in the Haber process produce more ammonia?
Answer:
- Yes. Increasing the pressure shifts the equilibrium to the right (towards fewer gas molecules), favoring the production of ammonia (NH₃).
c. What would be the effect of increasing the temperature in the Haber process on the level of ammonia produced?
Answer:
- Decrease in Ammonia Yield: Since the forward reaction is exothermic, increasing temperature shifts equilibrium to the left (favoring reactants), resulting in less ammonia production.
d. Why are the unreacted gases re-circulated?
Answer:
- Economic: Enhances the overall efficiency and cost-effectiveness of the process by minimizing the need for additional raw materials.
- Efficiency: Re-circulating unreacted nitrogen and hydrogen gases maximizes ammonia yield by allowing more reactants to be converted into ammonia without wasting raw materials.
Quizzes:
Quiz 1
Quiz 2